Synthesis and characterization of GBP@HA NPs
We prepared and characterized GBP@HA NPs based on our conceptual framework (Scheme 1a). As shown in Fig. 1a, the FT-IR Fig of BSA v = 1661 cm− 1, v = 1537 cm− 1 and v = 1239 cm− 1 are attributed to the amide (I) peak, amide (II) peak and amide (III) peak, respectively. In the spectrum of G-BSA, v = 1655 cm− 1, v = 1542 cm− 1 and v = 1247 cm− 1 are amide I, II, III bands, respectively, glycosylation can cause the carboxyl group in the lactobionic acid molecule and the free amino group in BSA for amide reaction, so its amide I, II, III band changes, 1135 cm− 1 in lactobionic acid corresponds to the -OH group expansion vibration in the galactosyl group, and the peak wavenumber in G-BSA is 1162 cm− 1, indicating the successful synthesis of G-BSA.
Then analyze its crystal structure through XRD. XRD spectra (Fig. 1b) showed that the broad peaks of lactobionic acid and BSA showed that both were amorphous, and physical mixing had no effect on them, and they were still amorphous, while there were obvious diffraction peaks at 31°(2θ) and 45°(2θ) in the spectra of G-BSA, indicating that G-BSA was crystalline, further proving the successful synthesis of G-BSA.
Analyze the synthesis process of GBP@HA NPs through potential changes. The potential change results of Fig. 1c show that the zeta surface potential of G-BSA-polyphenol NPs during the synthesis of GBP@HA NPs is -30.97 ± 0.29 mV. Because EPL has a positive charge and HA has a negative charge, G-BSA-polyphenol NPs are encapsulated layer by layer through EPL and HA to form GBP@HA NPs. In this process, GBP@EPL NPs are formed after EPL encapsulation, and the potential changes from − 30.97 ± 0.29 mV to 21.50 ± 0.53 mV, and then through HA encapsulation, it finally becomes − 22.23 ± 3.62 mV.
The circular dichroism patterns of BSA, G-BSA and G-BSA-polyphenol NPs (Fig. 1d) were verified and analyzed as shown in Table S1 (Supplementary Material), when BSA was modified by glycosylation, the content of its α-helix structure increased from 32.2%, the content of β-fold increased from 30.7 to 34.0%, and the content of β-corner decreased from 6.2 to 2.6%, indicating that the content of lactobonic acid glycosylation modified BSA The molecular secondary structure was affected, confirming the synthesis of G-BSA, but did not cause significant changes in the secondary structure. The binding of polyphenols to G-BSA further affects the structure of G-BSA, indicating that its binding may be related to hydrogen bonding, and cause secondary structural changes by affecting the intramolecular hydrogen bonding force of G-BSA.
We also tested the particle size distribution of GBP@EPL NPs and GBP@HA NPs, both of which showed a unimodal distribution. The particle size of GBP@EPL NPs was 367.57 ± 27.17 nm (Figure S1), with a PDI of 0.207. As shown in Fig. 1e, the particle size of GBP@HA NPs is 441.93 ± 17.27 nm with a PDI of 0.268. Both types of nanoparticles have good dispersibility and uniform distribution.
Stability and in vitro release profiles of GBP@HA NPs
Transmission electron microscopy was used to observe the morphology of nanoparticles, as shown in Fig. 1f-h, and it can be observed that the nanoparticles are regular and uniformly spherical, and the particle size distribution is about 500 nm, which is consistent with the particle size distribution detection results. To evaluate the stability of GBP@HA NPs, they were incubated in pepsin-containing artificial gastric juice and trypsin-containing artificial small intestinal fluid, respectively, and then the morphology of GBP@HA NPs was observed. Transmission electron microscopy showed that GBP@HA NPs were still relatively complete spherical after 3 h incubation in artificial gastric juice and artificial small intestinal fluid, and there is no significant change in particle size, so GBP@HA NPs had good stability in pepsin and trypsin, which was conducive to their smooth reaching of the colon site after oral administration.
The release curves of GBP@HA NPs under different pH conditions are shown in Fig. 1i-j, and the cumulative release rates of TA and EGCG in GBP@HA NPs within 48 h at pH 1.5 are (30.84 ± 1.24) % and (29.96 ± 0.43) %, respectively, indicating that they are more stable and less released under acidic conditions. At pH 6.8, the cumulative release rates of TA and EGCG increased, (68.84 ± 0.68) % and (38.29 ± 1.40) %, respectively, and the cumulative release rates reached (91.26 ± 0.42) % and (47.15 ± 0.90) % at pH 7.4, respectively, compared with the cumulative release rates at pH 1.5 and pH 7.4. Therefore, GBP@HA NPs can effectively resist the acidic environment, maintain good stability when administered orally, and carry out a good drug release process in the colon.
In vitro free radical scavenging ability of GBP@HA NPs
PTIO• is commonly used ROS radicals, DPPH• and ABTS•, commonly used reactive nitrogen species (RNS) radicals, and the antioxidant capacity of GBP@HA NPs was preliminarily tested by free radical scavenging experiments. As shown in Fig. 1k-m, the scavenging rate of GBP@HA NPs in the range of 50 ∼ 500 µg/mL is more than 60% for PTIO radicals, more than 70% for DPPH radicals, and more than 98% for ABTS radicals, and its clearance gradually increases with the increase of concentration, so GBP@HA NPs can effectively remove ROS and RNS, indicating that they may have good antioxidant function. At the same time, according to the detection results, 200 µg/mL was selected for cell experiments.
Synthesis and characterization of GBP@HA NPs. (a) Infrared spectra of G-BSA. (b) XRD spectrum of G-BSA. (c) Potential changes during the synthesis of GBP@HA NPs. (d) Circular dichroic spectra of BSA, G-BSA, and G-BSA-polyphenol NPs. (e) Particle size distribution of GBP@HA NPs. (f) Observe the morphology of GBP@HA NPs through transmission electron microscopy (pH = 7) and GBP@HA NPs were incubated in artificial gastric juice at pH 1.5 (g) and artificial intestinal juice at pH 6.8 (h) for 3 h before morphological observation. Release curves of (i) TA and (j) EGCG in GBP@HA NPs under different pH conditions (n = 3). The (k) PTIO, (l) DPPH, and (m) ABTS free radical clearance rates of GBP@HA NPs. All data are presented as mean ± SD
In vitro and in vivo biocompatibility of GBP@HA NPs
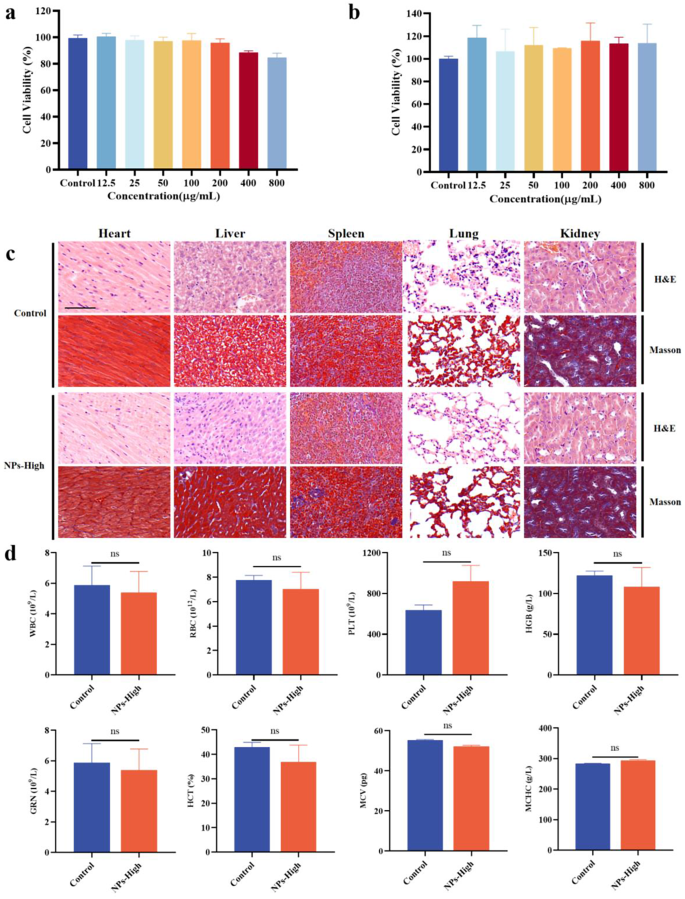
The safety of a drug is crucial to the application of the drug. Therefore, the cytotoxicity of GBP@HA NPs in RAW 264.7 mouse macrophages and Caco-2 colorectal cancer cells was first analyzed by MTT. As shown in Fig. 2a-b, at a concentration of 12.5 ∼ 800 µg/mL, the survival rate of RAW 264.7 macrophages and Caco-2 colorectal cancer cells was above 80%, and there was no obvious cytotoxicity, which proved that GBP@HA NPs had good in vitro biocompatibility. As ideal nanoparticles for oral administration, in addition to good therapeutic effects, good biocompatibility is also necessary. In this study, the heart, liver, spleen, lungs and kidneys of mice continuously given high doses of GBP@HA NPs were subjected to histopathological observation by H&E staining and Masson staining, and the blood cells of mice were analyzed and compared with normal mice.
As shown in Fig. 2c, cardiomyocytes are complete and neatly arranged, liver cells are normal and closely arranged, the structure of white pulp and red pulp in spleen tissue is clear and normal, there is no alveolar damage in the lungs, no inflammatory cells are produced, and the kidney tissue presents normal tubules and glomeruli, all without inflammatory reaction. The blood cells of mice were analyzed to detect white blood cells (WBC), red blood cells (RBC), platelets (PLT), hemoglobin (HGB), granulocytes (GRN), hematocrit (HCT), mean volume of red blood cells (MCV), and mean hemoglobin concentration (MCHC) in the blood of mice, and the results of Fig. 2d showed that there was no significant difference between the blood cells in the blood of mice in the NPs-High group and those of normal mice. The above results show that GBP@HA NPs have no adverse effects on the tissues and blood of mice, have good biocompatibility, and can be used as a safe delivery system to play a therapeutic role.
Effect of different concentrations of GBP@HA NPs on cell viability after incubation with (a) RAW 264.7 cells and (b) Caco-2 cells for 24 h, respectively. (n = 3) (c) H&E staining and Masson staining of heart, liver, spleen, lung, kidney of mice administered GBP@HA NPs mice (Scale bar: 200 μm). (d) Blood cell analysis of mice administered GBP@HA NPs (n = 3; ns: No significant difference). All data are presented as mean ± SD
Anti-inflammatory, antioxidant and cellular uptake properties of GBP@HA NPs
Cytokines play an irreplaceable role in the occurrence and development of inflammation, in which macrophages are closely related to the production of cytokines [56]. The anti-inflammatory effects of GBP@HA NPs in vitro were analyzed by investigating the secretion of four inflame-related cytokines, TNF-α, IL-1β, IL-6 and IL-10 by RAW 264.7 macrophages in different treatment groups. As shown in Fig. 3a, after the stimulation of LPS, the content of pro-inflammatory factors TNF-α, IL-1β and IL-6 increased significantly, while the content of anti-inflammatory factor IL-10 was significantly reduced. After treatment with mixed polyphenols, G-BSA-polyphenol NPs and GBP@HA NPs, the pro-inflammatory factors of each group were reduced and the anti-inflammatory factors were increased, among which the regulatory effect of GBP@HA NPs was the most significant, showing good in vitro anti-inflammatory effects.
We evaluated the ability of GBP@HA NPs to repair oxidative stress damage caused by H2O2 using MTT assay. As shown in the Fig. 3b, H2O2 will cause significant oxidative stress damage to cells, leading to a significant decrease in cell survival rate. Polyphenols can effectively repair oxidative stress damage and increase cell survival rates to be similar to normal cells. The formation of GBP@HA NPs has no significant effect on the repair effect of polyphenols, and can still significantly improve cell survival rates.
ROS in cells was detected by DCFH-DA fluorescence probe. Figure 3c shows that, after LPS stimulation, intracellular fluorescence was significantly enhanced and ROS content increased significantly. The fluorescence in cells treated with EGCG and TA mixed polyphenols, G-BSA-polyphenol NPs and GBP@HA NPs was significantly reduced, which proved that the growth of ROS content was inhibited. And We speculate that the antioxidant capacity of G-BSA-polyphenol NPs and GBP@HA NPs mainly came from natural polyphenols with good antioxidant effect, while the formation of G-BSA-polyphenol NPs and GBP@HA NPs can still maintain their antioxidant effect.
The targeting of GBP@HA NPs to macrophages was evaluated in vitro by cell uptake experiments. Fluorescence pictures showed that the fluorescence in normal macrophages without LPS treatment was weak, indicating that fewer GBP@HA NPs were ingested. And the fluorescence intensity in LPS-induced inflammatory macrophages was significantly enhanced than that of normal macrophages, indicating that the uptake of GBP@HA NPs by inflammatory macrophages was significantly increased, and GBP@HA NPs had good targeting of inflammatory macrophages (Fig. 3d). To further explore the targeting of GBP@HA NPs, we pretreated inflammatory macrophages with a mixture of lactobionic acid, HA, and lactobionic acid and HA, respectively, and then incubated with GBP@HA NPs, respectively. As shown in the Fig. 3d, the pretreatment of lactobionic acid and HA can weaken the fluorescence in inflammatory macrophages, and the mixture pretreatment of lactobionic acid and HA significantly reduces the fluorescence to be close to that of normal cells, which indicates that the good targeting of GBP@HA NPs to inflammatory macrophages is related to the galactose group and HA contained in GBP@HA NPs. Galactose groups and HA selectively bind to macrophages galacto-type lectins (MGL) and CD44 proteins that are highly expressed on the surface of inflammatory macrophages, respectively, promoting targeted binding of GBP@HA NPs to inflammatory macrophages and entry.
(a) The contents of TNF-α, IL-1β, IL-6 and IL-10 secreted by RAW 264.7 cells were detected by ELISA (n = 3; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). (b) The repair ability of GBP@HA NPs for oxidative stress damage caused by H2O2 was detected by MTT method in Caco-2 cells. (c) Detection of reactive oxygen species in RAW 264.7 cells using DCFH-DA fluorescence probe (scale: 50 μm). (d) Uptake of GBP@HA NPs by RAW 264.7 cells (blue fluorescence: DAPI-stained nucleus; Green fluorescence: Rhodamine B labeled GBP@HA NPs; White scale: 50 μm; Green scale: 25 μm). All data are presented as mean ± SD
GBP@HA NPs alleviate acute UC
As shown in Fig. 4a, after free drinking of 2.5% DSS aqueous solution for 8 d, the model group mice had severe diarrhea, and the feces were bloody mucus-like, the mobility was significantly reduced, and the coat color was visible to the naked eye. It showed that the mouse ulcerative colitis model was successfully constructed. In Fig. 4b, the blood in the stool of mice in each group was observed with naked eye, and it was seen that there were obvious traces of blood in the stool at the anus of the mice in the model group.
The weight of mice with colitis decreased significantly, and after treatment, the weight of five groups of mice in polyphenol group, G-BSA-polyphenol group, NPs-Low group, NPs-High group and 5-ASA group all recovered, among which the NPs-High group had the best therapeutic effect, indicating that GBP@HA NPs could effectively enhance the therapeutic effect of polyphenols, and the therapeutic effect was concentration-dependent (Fig. 4c). The DAI scores of mice in each group also demonstrated this result, with DSS-induced acute UC leading to a significant increase in the DAI score of the mice, and a decrease in DAI in all treatment groups, as well as the NPs-High group (Fig. 4d). At the same time, under the treatment effect of each group, the blood in the stool caused by colitis in mice was also significantly improved (Fig. 4b). This result was also confirmed in the acute UC model constructed through TNBS (Fig. S2a-c).
In addition, both DSS and TNBS induced mice developed symptoms of colitis, which developed colon congestion and swelling, weight gain, and shortened colon length. In addition, as an important component of the immune system, the inflammatory response can also affect the weight of the thymus and pancreas, so colon length, thymus weight, and spleen weight are important indicators of UC severity. As shown in the Fig. 4e-f and Fig. S2d-e, the colon of the model group mice was swollen and the length was significantly shortened, while the high dose of GBP@HA NPs had a significant protective effect on the colon, and the protective effect was better than that of the polyphenol group and the G-BSA-polyphenol group. For spleen changes caused by colitis, all groups of mice improved after treatment, and played a good alleviation effect on spleen enlargement caused by inflammation, and like other indicators, GBP@HA NPs also played a concentration-dependent therapeutic effect (Fig. 4g-h). Therefore, we found that compared with unmodified polyphenols, GBP@HA NPs have a good therapeutic effect on DSS-induced colitis, and can effectively improve the therapeutic effect of polyphenols, which is closest to that of normal mice.
Construction of a mouse model of ulcerative colitis and the therapeutic effect of GBP@HA NPs on UC. (a) Schematic diagram of experimental time. (b) Visual observation of mouse fecal blood situation image. (c) Changes in body weight of mice. (d) DAI score of mice. (e) Picture of mouse colon. (f) Colon length in mice. (g) Picture of mouse spleen. (h) Spleen index of mice. (i–l) The regulatory effects of GBP@HA NPs on antioxidant-related enzymes MDA, SOD, CAT and GSH-PX in colon tissues of UC mice. (m–r) The regulatory effect of NPs on inflammatory related factors MPO, TNF-α, IL-6, IL-1β and IL-10 in colon tissue of UC mice. (n = 6; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). All data are presented as mean ± SD
In addition, we evaluated the anti-inflammatory and antioxidant effects of GBP@HA NPs in mice with acute UC [57, 58]. The antioxidant capacity of GBP@HA NPs in vivo was analyzed by detecting the levels of MDA, SOD, CAT and GSH-Px in the colons of mice in each group. As shown in the Fig. 4i, DSS-induced colitis led to a significant increase in MDA content in the colon of mice, while the MDA content in each treatment group decreased compared with it, among which the NPs-Low group and NPs-High group were significantly reduced and concentration-dependent. After testing, we found that colitis significantly caused the reduction of SOD, CAT and GSH-Px antioxidant enzymes, and the levels of antioxidant enzymes were significantly increased after the treatment of polyphenols, G-BSA-polyphenols, low-dose GBP@HA NPs, high-dose GBP@HA NPs and 5-ASA, as shown in the Fig. 4j-l, the levels of SOD enzymes in the NPs-High group increased significantly. The CAT levels of NPs-Low group and NPs-High were significantly increased, and the GSH-Px levels of each treatment group were significantly increased, so GBP@HA NPs can effectively enhance the antioxidant effect of polyphenols to promote the improvement of colitis. The experimental results of TNBS induced acute UC in mice are consistent with them (Fig. S2f-i).
To analyze the anti-inflammatory effects of GBP@HA NPs in vivo, we examined the inflammation-related factors MPO, TNF-α, IL-6, IL-1β, and IL-10 in mouse colons (Fig. 4m). MPO is a functional marker and activation marker of neutrophils, and the occurrence of inflammation is closely related. As shown in the Fig. 4n-r, the content of MPO in mice with DSS induced acute UC is significantly increased compared with normal mice, and after drug treatment, the content of each group shows a significant downward trend, and the G-BSA-polyphenol group, NPs-Low group, NPs-High group and 5-ASA group are significantly reduced. The inflammatory factors TNF-α, IL-6 and IL-1β were significantly increased when inflammation occurred in the colon, but showed a significant decrease under the treatment effect of each group. And the content of anti-inflammatory factor IL-10 in normal mice was higher than that in the model group, the contents of the G-BSA-polyphenol group, NPs-Low group, NPs-High group and 5-ASA group were significantly higher than those in the model group. The detection of inflammatory factors in the colon of TNBS induced acute colitis mice further confirms that GBP@HA NPs have a regulatory effect on inflammatory factors and can also play a role in reducing MPO (Fig. S2j-n). The results of these inflammation-related factors showed that GBP@HA NPs had good anti-inflammatory effects in vivo and significantly enhanced the anti-inflammatory effects of polyphenols.
Histopathological evaluation
The colons of DSS and TNBS induced acute colitis mice were stained with hematoxylin and eosin (H&E) and Masson for histopathological analysis (Fig. 5 and Fig. S3a). As shown in the Fig. 5a, each layer of the intestinal wall of the colon of DSS-induced colitis mice had different degrees of inflammatory cell infiltration, intestinal mucosal defects and crypt structure damage. The pathological injuries of mice in the treatment group were improved to varying degrees, among which the G-BSA-polyphenol NPs group, NPs-Low group and NPs-High group were the closest to the normal colon, and the histopathological scores of each group also confirmed this result. At the same time, the collagen fibers of the tissue were stained by Masson staining, which further confirmed the pathological changes in the colon tissue. As shown in the Masson staining image, the colonic tissue structure of mice in the model group was destroyed, accompanied by collagen deposition and fibrosis, and the treatment of each group had an inhibitory effect on colon tissue structure damage, collagen deposition and fibrosis, and promoted colon recovery, among which the G-BSA-polyphenol NPs group, NPs-Low group and NPs-High group had the best treatment effect and was closest to normal tissue.
In addition, the analysis of colon tissue by alcian blue staining can observe the mucus matrix of colon tissue. Intestinal cells secrete mucus to cover the epithelial mucosal layer, forming the intestinal mucosal barrier, and the mucus contains IgA and antimicrobial peptides that work together to defend against the invasion of harmful commensal bacteria and pathogens. Figure 5b shows that colitis in the model group leads to the destruction of colonic tissue, a significant reduction in mucus secretion, and damage to the intestinal mucosal barrier. Compared with the model group, mucus secretion in colon tissues was significantly increased in all treatment groups, and the G-BSA-polyphenol NPs group, NPs-Low group and NPs-High group recovered to be close to normal mice. Therefore, compared with natural polyphenols and 5-ASA, the modified GBP@HA NPs can effectively inhibit the pathological damage of the colon caused by colitis and repair the intestinal mucosal barrier.
Histopathological observation. (a) H&E staining and Masson staining of colon tissue in each group of mice. (b) Alcian blue staining of colon tissue in each group of mice. (c) Cy7 labeled GBP@HA NPs were administered orally to normal group mice and UC model group mice, and whole body imaging was performed using IVIS technology at 4 h, 6 h, 12 h, and 24 h. (d) The heart, liver, spleen, lungs, and kidneys, as well as the digestive tract from the stomach to the colon, were collected to detect the fluorescence distribution of each site. All data are presented as mean ± SD. (n = 3; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Magnification: 20 ×; Scale: 200 μm)
Targeting and biodistribution of GBP@HA NPs in colitis mice
Next, we analyzed the in vivo distribution of GBP@HA NPs using Cy7 fluorescent dye. Figure 5c shows that significant fluorescence was observed in both normal mice and UC model mice after administration, and the fluorescence decreased over time. However, the fluorescence in normal mice decreased more significantly. According to the fluorescence distribution maps in various organs (Fig. 5d), during the 24-hour distribution process, there was no fluorescence distribution in the liver, liver, spleen, lungs, and kidneys, but only in the gastrointestinal tract. After 12 h, UC mice showed stronger fluorescence in the gastrointestinal tract than normal mice, and there was still significant fluorescence in the colon after 24 h, indicating that GBP@HA NPs have good colon targeting ability.
Intestinal barrier repair and regulation of macrophage polarization
The presence of tight junctions between intestinal epithelial cells in the intestinal barrier is essential and plays an irreplaceable role in maintaining the integrity of the intestinal barrier, and the expression of tight junction proteins between intestinal epithelial cells is affected when colitis occurs, resulting in changes in the structure and function of tight junctions, so the detection of tight junction proteins is an important part of evaluating the therapeutic effect of colitis. By dual immunofluorescence staining, we analyzed the four tight junction proteins ZO-1 and Occludin, Claudin-4, and E-Cadherin, respectively. The immunostaining results (Fig. 6a-b and Fig. S3b) showed that the fluorescence of ZO-1, Occludin, Claudin-4 and E-Cadherin in the colon of normal mice was strong, which proved that the content of tight junction protein was high and the intestinal barrier function was intact. However, the intestinal barrier function of mice with colitis was impaired, tight junctions were destroyed, the content of related proteins was significantly reduced, and the fluorescence intensity was significantly weakened. Through the treatment of GBP@HA NPs and 5-ASA, the fluorescence intensity of the four proteins was enhanced compared with the model group, that is, the content of the four proteins increased, the intestinal barrier function was restored. And the fluorescence intensity of the GBP@HA NPs treatment group was also stronger than that of the 5-ASA group, which was close to that of the normal group, indicating that GBP@HA NPs can improve the colon condition of mice and treat colitis by repairing the intestinal barrier.
Macrophages, as immune cells that play an important role in the intestinal immune barrier, can effectively help maintain and regulate intestinal homeostasis [59]. Macrophages in different environments can be polarized into M1 (classical activation) and M2 (selective activation) two different phenotypes, M1 macrophages have pro-inflammatory effects, in the process of colitis occurrence and development of M1 macrophages polarization increases, causing a further increase in pro-inflammatory cytokines, while M2 has anti-inflammatory effects, can promote the secretion of anti-inflammatory factors, and is related to the resolution of inflammation [60]. In this study, the markers iNOS (inducible nitric oxide synthase) and CD206 of M1 and M2 macrophages were selected as indicators for the evaluation of macrophage polarization, as shown in Fig. 6c, iNOS (red fluorescence) expression was very little, while CD206 (green fluorescence) expression was significantly more than iNOS, indicating that macrophages in normal mice were multipolarized to M2 type. In contrast, iNOS expression was significantly higher than CD206 in the colon tissues of DSS-induced mice with colitis, indicating that colitis development is closely related to increased polarization of M1-type macrophages. After treatment with GBP@HA NPs and 5-ASA, the expression of iNOS in mouse colon tissues decreased, and the expression of CD206 in the NPs-High group increased significantly compared with the model group, indicating that regulating the M1 and M2 polarization of macrophages, reducing M1 cells, and increasing M2 cells is another important reason why GBP@HA NPs can effectively play a therapeutic role.
Study on repairing effect of intestinal barrier and regulating macrophage polarization by GBP@HA NPs. (a) Immunofluorescence staining and quantitative analysis of ZO-1 and Occludin in the colon (Blue fluorescence: DAPI-stained nucleus; Green fluorescence: ZO-1; Red fluorescence: Occludin) (b) Immunofluorescence staining and quantitative analysis of Claudin4 and E-Cadherin in the colon (Blue fluorescence: DAPI-stained nucleus; Green fluorescence: Claudin4; Red fluorescence: E-Cadherin). (c) Immunofluorescence staining and quantitative analysis of iNOS and CD206 in the colon (Blue fluorescence: DAPI stained nuclei; Green fluorescence: CD206; Red fluorescence: iNOS) scale: 400 μm; magnification: 40 ×
GBP@HA NPs regulate the intestinal flora
Important influences of the gut microbiota have been identified in maintaining health as well as disease pathogenesis [61, 62]. In this study, the intestinal bacteria in the feces of each group of mice were analyzed. First, the richness and diversity of the microbiota were analyzed, mainly evaluating the α-diversity index: operational taxa (OTUs), Chao1 index and Simpson index. As shown in Fig. 7a, the OTUs and Chao1 indices of DSS-induced colitis mice were significantly lower than those of normal mice, indicating that colitis affected intestinal homeostasis and reduced the abundance of intestinal flora, while the Simpson index of colitis mice was significantly reduced, indicating a decrease in the diversity of intestinal flora in mice with colitis. After the treatment of polyphenols and GBP@HA NPs, the OTUs, Chao1 index and Simpson index of mice were increased, indicating their recovery effect on the abundance and diversity of intestinal flora, among which GBP@HA NPs could promote the significant increase of OTUs, Chao1 index and Simpson index in mice with colitis, indicating its significant role in restoring the abundance and diversity of intestinal flora.
A detailed analysis of the microbiota was conducted at the phylum and genus levels (Fig. 7b). The main intestinal bacteria were analyzed at the phylum and genus levels, respectively. As shown in Fig. 7c, tests were performed at the genus level, and the main intestinal bacteria genera among them were analyzed. Lactobacillus is the most abundant probiotic in the gut, which is conducive to maintaining intestinal homeostasis and immune conditions [63]. Akkermansia, a normal human intestinal flora, not only plays a metabolic protective role in protecting the integrity of intestinal epithelial cells and mucus layer, but also plays an anti-inflammatory role in the process of inflammation through regulatory T cells, endocannabinoid system and non-classical toll-like receptors [64]. Studies have shown that enterotoxins produced by the genus Bacteroides are present in the human gut during active colitis and are closely associated with colitis [65]. Klebsiella has a symbiotic relationship with humans, helping to break down food and produce the nutrients and energy the body needs. However, when the immune system is damaged or the intestinal barrier is damaged, Klebsiella enter parts of the body other than the gastrointestinal region, which can cause infection, oxidative stress and other adverse effects [66]. As shown in Fig. 7d, in the intestines of normal mice, Lactobacillus and Akkermania glutinophilus were abundant, Klebsiella and Bacteroides were abundant, and the opposite was true in colitis mice. The regulatory effect of GBP@HA NPs changes the flora abundance, which is close to that of normal mice. Figure 7e-f shows that at the phylum level, Bacteroidetes, Firmicutes, and Proteobacteria have the highest abundance. In colitis mice, Bacteroidetes increased while Firmicutes decreased. The Firmicutes/Bacteroidetes ratio (F/B), as an important indicator reflecting the degree of intestinal microbiota disorder, decreased compared to normal mice. After treatment with GBP@HA NPs, the F/B ratio of mice significantly increased, confirming the role of GBP@HA NPs in regulating intestinal microbiota and inhibiting intestinal disorders. The results of principal coordinate analysis (PCoA) (Fig. 7g) were consistent with it, and the distance between the control group and the model group was the farthest, indicating that the difference between the two groups was the greatest. GBP@HA NPs treatment could reduce the difference between the colitis mice and the control group, that is, close to normal mice. Therefore, GBP@HA NPs can effectively regulate intestinal flora and restore intestinal homeostasis.
The role of GBP@HA NPs in regulating gut microbiota. (a) Study the effect of GBP@HA NPs on the abundance and diversity of gut microbiota through OTUs, chao1 index, and Simpson index. (b) Heat map of gut microbiota at the phylum and genus levels. (c) The relative abundance of gut microbiota at the genus level. (d) The relative abundance of important genera in the gut microbiota. (e) The relative abundance of gut microbiota at the gate level. (f) Ratio of Firmicutes/Bacteroidetes ratio (f/B). (g) Principal coordinate analysis (PCoA) diagram of intestinal microbiota. (n = 6; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). All data are presented as mean ± SD
GBP@HA NPs alleviate chronic UC
Inspired by the therapeutic effect of GBP@HA NPs on acute UC, we further explored its therapeutic effect in DSS induced chronic UC mouse model (Fig. 8a). As shown in the Fig. 8b, the weight of chronic UC mice induced by DSS was significantly reduced compared to normal mice. After 7 weeks of treatment, the polyphenol group, G-BSA-polyphenol group, NPs-Low group, NPs-High group, and 5-ASA group all showed higher body weight than UC mice, demonstrating a good weight recovery effect. Similarly, each treatment group also demonstrated therapeutic effects on colon length reduction caused by colitis, with the NPs-High group returning to a level close to that of the normal group (Fig. 8c-d). As shown in the Fig. 8e-f, GBP@HA NPs also has a good relief effect on spleen enlargement caused by colitis, and the spleen volume and index are significantly reduced. Next, the inflammation-related factors MPO, TNF-α, IL-6, IL-1β and IL-10 in the colon of mice in each group of the chronic UC model were analyzed. As expected, GBP@HA NPs can effectively reduce the increase of MPO, TNF-α, IL-6, IL-1β caused by chronic UC, and increase the level of anti-inflammatory factor IL-10 (Fig. 8g-k).
The therapeutic effect of GBP@HA NPs on chronic UC. (a) Schematic diagram of experimental time. (b) Changes in body weight of mice. (c) Picture of the mouse colon. (d) Mouse colon length. (e) Picture of mouse spleen. (f) Mouse spleen index. (g-k) The regulatory effect of NPs on inflammatory related factors MPO, TNF-α, IL-6, IL-1β and IL-10 in colon tissue of chronic UC mice. (n = 6; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). All data are presented as mean ± SD
The histopathological studies conducted in chronic UC models have further demonstrated the repair effect of GBP@HA NPs on UC-induced colon tissue damage. In addition, we conducted histopathological studies. The H&E staining results (Fig. 9a) indicate that chronic UC can also cause severe damage to colon tissue, causing problems such as crypt damage and inflammatory cell infiltration. GBP@HA NPs also demonstrate good therapeutic effects and effectively repair damaged colon tissue. Meanwhile, the Masson staining results (Fig. 9a) indicate that GBP@HA NPs can play a significant therapeutic role in promoting colon recovery in chronic UC induced collagen deposition and fibrosis in the colon. Therefore, GBP@HA NPs can also play an excellent role in inhibiting colonic pathological damage caused by colitis and repairing the intestinal mucosal barrier in chronic UC.
To investigate whether GBP@HA NPs can alleviate colonic intestinal barrier damage caused by chronic UC, we detected tight junction protein by immunofluorescence staining. As shown in Fig. 9b-c, GBP@HA NPs showed a good therapeutic effect on the reduction of tight junction proteins ZO-1, Occludin, Claudin4 and E-Cadherin caused by chronic UC, and the fluorescence of the four tight junction proteins was significantly enhanced, indicating a significant increase in their content. The damaged intestinal barrier is repaired. We also studied the regulatory effect of GBP@HA NPs on macrophage polarization by immunofluorescence staining.
Study on repairing effect of intestinal barrier by GBP@HA NPs. (a) H&E staining and Masson staining of colon tissue in each group of mice. (Magnification: 20 ×) (b) Immunofluorescence staining and quantitative analysis of ZO-1 and Occludin in the colon (blue fluorescence: DAPI-stained nucleus; Red fluorescence: ZO-1; Green fluorescence: Occludin) (c) Immunofluorescence staining and quantitative analysis of Claudin4 and E-Cadherin in the colon (blue fluorescence: DAPI-stained nucleus; Red fluorescence: Claudin4; Green fluorescence: E-Cadherin). Scale: 400 μm; Magnification: 40 ×
The regulatory effect of GBP@HA NPs on metabolites
We used UPLC-Q-MS technology to detect endogenous metabolites in mouse colon tissue, preliminarily screening differential metabolites related to GBP@HA NPs alleviating colitis, and conducting a deeper exploration of their therapeutic mechanisms. As shown in Fig. 10a-d, the blank control group and model group were significantly separated, indicating a change in the metabolic level of colonic tissue content in mice after modeling. There was no aggregation between the model group and the GBP@HA NPs group, indicating a good separation. This indicates that the metabolic level of colonic content in mice with colitis also changed after treatment with GBP@HA NPs. Figure 10e shows that colitis causes significant changes in metabolites in mice, while treatment with GBP@HA NPs reduces the differential metabolites between colitis mice and normal mice. Metabolites such as L-tryptophan, phenylacetylglycine, biochanin A, hepoxilin A3 and 5-hydroxyindoleacetic acid in the colon of model group mice were significantly increased compared to normal mice and L-Asparagine, glycitein, epiandrosterone, and L-Aspartic acid metabolites were significantly downregulated (Fig. 10f). Further pathway enrichment analysis of differential metabolites between the GBP@HA NPs group and the model group showed that the amino acid metabolism pathway is affected, and amino acids not only provide energy, but their metabolites can also participate in important physiological activities (Fig. 10g-h). Cytoplasmic glutamate is crucial for maintaining redox balance and avoiding oxidative stress in cells by producing glutathione (GSH). We also found that UC development is associated with the peroxisome proliferator-activated receptor (PPAR) signaling pathway, a ligand-activated receptor in the nuclear hormone receptor family that plays an important role in inflammatory responses and binds to the NF-κB subunit that regulates the expression of inflammatory factors [67]. Therefore, based on its therapeutic efficacy and metabolomics research, a deeper exploration of its potential mechanisms has been conducted.
(a) PLS-DA score plot between the control group and the model group under positive ion mode. (b) PLS-DA score plot between the control group and the model group under negative ion mode. (c) PLS-DA score plot between GBP@HA NPs group and model group in positive ion mode. (d) PLS-DA score plot between GBP@HA NPs group and model group in negative ion mode. (e) Differential metabolite statistics. Red: increase the number; Blue: Decreased number. (f) Z-score heat map of differential metabolites. MetaboAnalyst was used to perform KEGG pathway enrichment analysis on differential metabolites between the GBP@HA NPs group and the model group. (g) Difference enrichment score chart. (h) Scatter plot for differential metabolite enrichment analysis
Potential mechanisms by which GBP@HA NPs alleviate colitis
Studies have shown that oxidative stress caused by oxidation and antioxidant imbalance in the body affects the occurrence and development of UC [68]. The Keap1-Nrf2 signaling pathway is a key pathway in cellular oxidative stress, and the mRNA transcription and expression of Keap1 and Nrf2 in the colon tissues of each group of mice were analyzed by qPCR and Western blotting [69, 70]. As shown in the Fig. 11a-b, under oxidative stress, the mRNA levels of Keap1 and Nrf2 in the colon tissues of colitis mice were significantly increased compared with normal mice, and the treatment groups played a regulatory role, so that their relative expression levels were significantly reduced. Western blotting’s detection of Keap1 and Nrf2 proteins also confirmed this result, and the regulation of GBP@HA NPs was the most obvious, and the NPs-High group decreased to close to the normal group (Fig. 11c). Therefore, the powerful regulation of Keap1-Nrf2 signaling pathway by GBP@HA NPs is an important way to alleviate oxidative stress and exert antioxidant effects.
Studies have found that NF-κB controls many genes related to inflammation, so the activation of NF-κB signaling pathway is also closely related to the inflammatory response in the colon [71]. The detection of NF-κB mRNA expression level showed that the expression level of NF-κB mRNA in colonic cells was significantly increased when colitis occurred, which was about 4 times that of normal mice and the G-BSA-polyphenol group and NPs-High group were significantly lower than the model group. At the same time, the mRNA expression levels of pro-inflammatory factors TNF-α, IL-1β, IL-6 and anti-inflammatory factor IL-10 were detected, as shown in the Fig. 11d-h, which was consistent with the detection results of Elisa. Reduced the increased mRNA levels of TNF-α, IL-1β, and IL-6 caused by colitis, and increased the expression of anti-inflammatory factor IL-10. In addition, Western blotting also presented the expression of four inflammation-related factors (Fig. 11i). As we imagined, under the regulation of GBP@HA NPs, TNF-α, IL-1β, IL-6. The expression levels were significantly down-regulated, while the expression level of IL-10 was significantly increased. These results suggest that GBP@HA NPs may effectively inhibit inflammatory response by regulating NF-κB signaling pathway.
In order to further explore the regulatory role of GBP@HA NPs on the cytopyrosis pathway, we analyzed the genes and proteins associated with cytopyrosis. First of all, immunofluorescence staining was applied to the colon site NLRP3 (red fluorescence), Caspase-1 (green fluorescence) and GSDMD (pink fluorescence) preliminary evaluation. As shown in the Fig. 11n, under normal state the three proteins are less, fluorescence intensity is weak, and the DSS-induced colitis mice colon three fluorescence in the colon is significantly enhanced, indicating that the three important proteins associated with cell pyroptosis are significantly increased, while the fluorescence of the GBP@HA NPs treatment group is significantly weakened, suggesting a close connection to the cytopyrosis pathway. Next, qPCR analysis results showed that the mRNA levels of NLRP3, Caspase-1 and GSDMD were consistent with the trend shown by fluorescent staining, and the mRNA levels of the three proteins were significantly reduced after each group of drug administration (Fig. 11j-l). The Western blotting band further confirmed this result (Fig. 11m). GBP@HA NPs exerted a powerful inhibitory effect on the increase of NLRP3, Caspase-1 and GSDMD expression in colitis, similar to that of normal mice. In summary, GBP@HA NPs can downregulate NLRP3, Caspase-1, and GSDMD, thus possessing the potential to regulate the cell pyroptosis pathway and alleviate colitis.
Exploration of the role of GBP@HA NPs in regulating signaling pathways. (a-b) RT-qPCR measured the relative expression levels of Keap1 and Nrf2 mRNA in the colon tissues of mice in each group. (c) Western blotting measured the expression of Keap1 and Nrf2 in the colon tissues of each group of mice. (d-h) RT-qPCR detected the relative expression levels of mRNA in NF-κB p65, TNF-α, IL-1β, IL-6 and IL-10 in the colon tissues of each group of mice. (i) Western blotting detected the expression of NF-κB p65, TNF-α, IL-1β, IL-6 and IL-10 in the colon tissues of mice in each group. (j-l) RT-qPCR was used to detect the relative mRNA expression levels of NLRP3, Caspase-1 and GSDMD in colon tissues of mice in each group. (m) Western blotting detected the expressions of NLRP3, Caspase-1 and GSDMD in colon tissues of mice in each group. (n) Immunofluorescence staining of NLRP3, Caspase-1, and GSDMD in the colon (Blue fluorescence: DAPI stained nuclei; Red fluorescence: NLRP3; Green fluorescence: Caspase-1; Pink fluorescence: GSDMD; Scale: 100 μm; Magnification: 40 ×). (n = 3; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). All data are presented as mean ± SD