Synthesis and characterization of the photocleavable polymer
The NIR-photocleavable polymer used in this study (PEGOD-EA-LMB) was synthesized based on a previously described protocol [51] with some modifications. In brief, 50 g of poly(ethylene glycol) methyl ether (mPEG; 5,000 Da; 81323; Sigma–Aldrich) was dissolved in 100 mL of toluene (8541 − 4100; Daejung; Siheung, South Korea) and subjected to azeotropic distillation until the volume was reduced to about 20 mL of visibly clear solution. Separately, 20 mmol of 4-nitrophenyl chloroformate (160210; Sigma–Aldrich) and 20 mmol of triethylamine (85556 − 4400; Daejung) were dissolved in 10 mL of dichloromethane (DCM; Samchun Chemicals, Seoul, South Korea) and added dropwise to the mPEG solution, which was stirred overnight at room temperature (RT). The mixture was slowly added to diethyl ether (4025–4400; Daejung) on ice with continuous stirring to precipitate the product. The precipitated product was purified by dissolution–precipitation with DCM and diethyl ether and recrystallized in ethyl acetate (4016 − 1100; Daejung). The PEG-nitrophenyl intermediary product was vacuum-dried. Next, 5 mmol of PEG-nitrophenyl was dissolved in 10 mL of DCM and added dropwise to a solution of octamethylene diamine (20 mmol) dissolved first in methanol (5558 − 4400; Daejung) and then in DCM. Triethylamine (2 mmol) in DCM was added (250 µL) to the solution and stirred overnight. The reaction mixture was precipitated in diethyl ether with dissolution–precipitation and recrystallization steps similar to the first intermediary product, PEG-nitrophenyl. The resulting product was PEGOD. The product was vacuum-dried and stored under reduced pressure prior to use.
Modified 10-N-carbamoyl MB was synthesized according to previously described protocols with some modifications [52] and conjugated to the synthesized PEGOD. The modified product LMB was dissolved in DCM (5 mmol) and added dropwise to a solution of PEGOD in DCM (2 mmol). Triethylamine (2 mmol) in DCM was added (250 µL) to the solution. The reaction was run overnight and precipitated, purified, and recrystallized, following protocols similar to those of the intermediary products. The resulting product, PEGOD-EA-LMB, was vacuum-dried and stored in the dark under reduced pressure.
The chemical structure of the synthesized PEGOD-EA-LMB was characterized by 1H NMR (sample dissolved in CDCl3, δ ppm: 7.24) using a Bruker AVANCE 600 (Bruker, Billerica, MA, USA) instrument. The molecular weight was calculated based on the ratio of end-group protons to polymer chain protons. FT- IR (6100 type A; JASCO, Tokyo, Japan) was also performed to confirm the synthesized polymer. A potassium bromide (KBr) disk containing 1.0 wt% of the sample was analyzed in the range of 4,000–400 cm− 1 at a resolution of 4 cm− 1 with 32 cumulative scans.
Fabrication of PCNs
Photocleavable nanoparticles were formed via the self-assembly mechanism in aqueous–organic–aqueous solutions followed by drying steps. First, 30 mg of PEGOD-EA-LMB polymer was dissolved in Dulbecco’s phosphate-buffered saline (DPBS; 20-031-CV; Corning, Jiangsu, China) containing different concentrations of either BSA (BioShop, Burlington, Canada: 5, 10, and 30 µg) or BDNF (788902; BioLegend, San Diego, CA, USA: 1, 5, and 10 µg), and freeze-dried. The powder was resuspended in ethyl acetate and vacuum-dried. The dried powder was reconstituted in PBS for subsequent analysis. Samples were kept in the dark at 4 °C to maintain stability. The generated nanoparticles were morphologically assessed by TEM (JEM 1400; JEOL, Tokyo, Japan). The % EE for BSA and BDNF in nanoparticles was calculated as (total BDNF added– free BDNF)/total BDNF added.
Light treatment of PCNs
A commercially available laser with a wavelength of 808 nm and frequency of 47–63 Hz (PSU-III-LED; CNI Laser, Changchun, China) was used according to the manufacturer’s instructions. UV (254 nm) exposure was performed using CL-1000 UV Crosslinker (UVP, Upland, CA, USA). Dosimetry was performed to determine laser settings before conducting laser treatment using a power meter probe and measuring the power output of the laser handpiece at a distance of 3.0 cm from the bottom of the plate (average penetration rate of 17% in a 4-week-old C57B6 mouse purchased from NARA Biotech (Seoul, South Korea)). The laser setting was adjusted to achieve a power density of 200 mW/cm2. Each laser shot was adjusted based on the irradiation time (150, 300, and 450 s) to achieve the specified energy densities (30, 60, and 90 J/cm2, respectively). The effect of laser treatment on the size of the PCNs was evaluated by dynamic light scattering using a zeta potential and particle size analyzer (ELSZ-2000; Otsuka Electronics, Osaka, Japan).
Evaluation of cytotoxic effects of PCNs and laser treatment on hNPCs
hNPCs (ACS-5004; ATCC, Manassas, VA, USA) were used to determine the cytotoxicity of PCNs in culture. To culture hNPCs, plates were coated with 1% cellular basement membrane (ATCC) for 1 h at RT before seeding. For the cytotoxicity evaluation of the PCNs, hNPCs were seeded in coated 96-well plates with a cell concentration of 2 × 104 cells/mL, cultured in recommended commercial hNPC growth medium (ACS-3003; ATCC), and maintained inside an incubator at 37 °C and 5% CO2. The hNPCs were grown and allowed to proliferate for 3 days prior to treatment.
Different concentrations of PCNs (500–1,000 µg/mL) were prepared by suspending the particles in the hNPC growth medium. Medium from the hNPCs grown in 96-well plates was removed, and PCNs were added to each well (100 µL) containing hNPCs kept for 24 h inside the incubator at 37 °C and 5% CO2 before cytotoxicity assay. Cytotoxicity assay was performed using CCK-8/EZ-Cytox assay (DoGenBio, Seoul, South Korea). The assay detects viable cells based on water-soluble tetrazolium salt (WST-8) reduced by dehydrogenase activities, giving a yellow formazan dye soluble in the tissue culture medium. The solution was mixed at a ratio of 1:9 with fresh medium and incubated for 4 h inside the incubator at 37 °C and 5% CO2. Optical densities were obtained at 450 nm using a microplate reader (Infinite 200 PRO; Tecan, Grödig, Austria). Triplicate readings were obtained from at least three individual culture and treatment batches. The data are reported as mean ± standard deviation. The same treatment schedule and protocols were followed for the determination of the potential cytotoxic effects of laser-only treatment and PCNs (500 µg/mL) at different energy densities (30, 60, and 90 J/cm2) in hNPCs for CCK-8 viability assay. PCNs suspended in PBS were centrifuged and resuspended in an hNPC growth medium. The PCNs were sonicated prior to use to remove agglomeration. Additional immunofluorescence staining was performed for PCNs with laser treatment. The hNPCs were stained with Alexa Fluor™ 488 Phalloidin (A12379; Invitrogen, Carlsbad, CA, USA), which selectively stains the filamentous actin (F-actin) of cells, with 4′,6-diamidino-2-phenylindole (DAPI; 5087410001; Sigma–Aldrich) used for nuclei staining. The samples were observed under a fluorescence microscope (EVOS™ M7000 Imaging System; Invitrogen, Darmstadt, Germany).
Effects of laser treatment and PCNs on hNPC differentiation
The amount of BDNF released (%) from PCNs upon irradiation at different energy densities (0, 30, 60, and 90 J/cm2) was determined using a Human BDNF ELISA Kit (ab212166; Abcam, Eugene, OR, USA) following the manufacturer’s protocol. The amount of PCNs needed to deliver approximately 100 ng of BDNF onto the cultured hNPCs during neuronal differentiation was calculated.
First, hNPCs in growth medium cultured for 3 days were treated with PCNs and laser irradiation. After treatment, the growth medium was changed to NDM without BDNF consisting of basal Dulbecco’s modified Eagle‘s medium (DMEM)/Ham’s F12 (10-090-CV; Corning, Corning, NY, USA), 1× N2 Supplement (17502-048; Gibco; ThermoFisher Scientific, Waltham, MA, USA), 2× B27 Supplement (17504-044; Gibco), 1% penicillin/streptomycin (30-2300; ATCC), 1,000 U/mL leukemia inhibitory factor (LIF; ESG1107; Millipore, St. Louis, MO, USA), and 10 ng/mL NT-3 (712102; BioLegend). BDNF was omitted from the medium to assess the effects on differentiation of BDNF released from PCNs alone. The hNPCs were kept for 7 days in culture and fixed for immunofluorescence staining. Images were acquired using the fluorescence microscope. The mean fluorescence intensity via obtained using ImageJ analysis of three batches of samples with an average of six images.
Primary vestibular ganglion culture
Vestibular ganglion isolation
Postnatal day 1 pup (P1; n = 22) of Sprague–Dawley rats were used in this study. We rinsed the pups’ head and neck parts with 70% ethanol and decapitated using surgical scissors. After opening the skin, using the tips of Iris scissors, an incision was made along the midline of the skull, starting from the brainstem area. The cerebrum part was lifted toward the brainstem to expose the ventral side of the brain by flipping it over using curved forceps. At this point, we can identify two nerve bundles connecting the brainstem and the inner ear (Fig. 7a). The end of the nerve bundles of the brainstem was cut using micro scissors, and then the inner ear was extracted in cold 1x HBSS (14025-092; Gibco). We can distinguish the vestibular ganglion on the extracted inner ear (Fig. 7a). The vestibular ganglions were isolated from the inner ear using fine forceps and collected in a 1.5 ml tube containing 1x HBSS (Gibco) [28].
Vestibular ganglion dissociation
The vestibular ganglions were transferred to another 1.5 ml tube containing prewarmed TrypLE™ Express Enzyme (12604-013; Gibco) and incubated in a 37 °C water bath for 20 min. After incubation, TrypLE™ Express Enzyme was removed, and the ganglion were washed using 1x HBSS three times. The ganglion was dissociated using pipette of 1000, 200, and 10 \(\:\mu\:l\). After dissociation, they were separated as single cells using 40 \(\:\mu\:m\) cell strainer (352340; Corning). Cell count was performed using Hematocytometer and Trypan Blue (15250-061; Gibco).
Vestibular ganglion organoids plating and differentiation
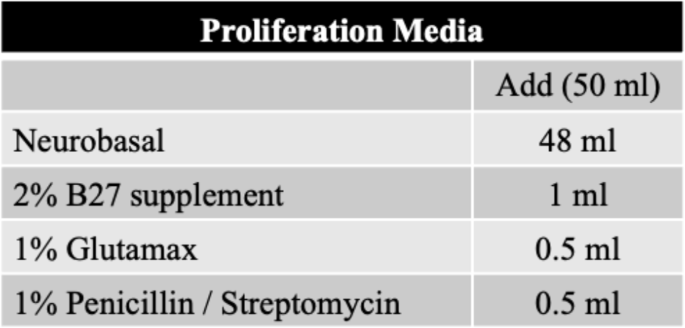
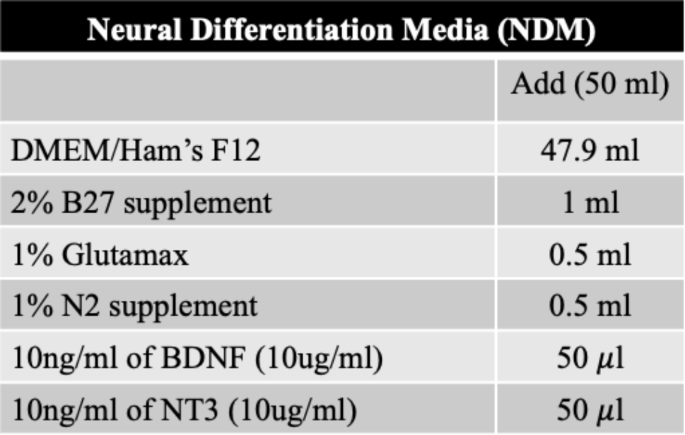
After dissociation, 3.5 × 104 of cells were seeded into a PrimeSurface 96 M 3D cell culture plate (MS9096UZ; S-BIO, NH, Hudson, USA). First two days of the culture are considered as -2 and − 1 day, which is for proliferation under proliferation media. The proliferation medium consisted of neurobasal medium (21103-049; Gibco) supplemented with 2% of B27 (17504-044; Gibco), 1% of GlutaMAX (35050-061; Gibco), and 5,000 U/mL penicillin/5,000 µg/mL streptomycin (Corning) (Table 1). In the proliferation media, cells begin to aggregate, and at this time, cells other than ganglia cells are degenerated due to the characteristic of proliferation media which allows the limited survival of the other cells [21,22,23]. In the primary VGNs culture, decreased number of cells are also observed at D0 compared to D-2 (S5). Once confluence was achieved or sufficient cells were aggregated, organoids were transferred to new 3D cell culture plate containing neural differentiation media (NDM). The NDM consisted of DMEM/Ham’s F12 (Corning) supplemented with 2% of B27 (Gibco), and 1% each of GlutaMAX (Gibco) and N2 (17502-048; Gibco). Additionally, 10 ng/mL each of BDNF (BioLegend) and NT-3 (BioLegend) were added for differentiation (Table 2). Medium change and differential interference contrast imaging was performed every 3 days. Primary VGNs culture was also performed. 9 × 104 of cells were seeded into 12-well plate including proliferation media with 18-mm cover glass coated by 0.5 \(\:\mu\:\)g/ml of Poly-D-Lysine (A3890401; Gibco).
Cells were fixed on D0, D3, and D21 for immunohistochemical evaluation to confirm the characteristics and differentiation of vestibular ganglion culture. Whole-cell patch clamp and vestibular organoid disease modeling were performed on D21, which was considered the complete differentiation stage in our culture system. Cells were grown in NDM without BDNF and treated with 1 mM ouabain (Sigma–Aldrich) for vestibular organoid disease modeling.
Immunofluorescence staining
Cells were fixed in 100% methanol or 4% paraformaldehyde and permeabilized with 0.3% or 0.1% Triton X-100 (T8787; Sigma–Aldrich) in 1 × PBS. After blocking in 10% or 3% BSA (ALB001; BioShop), cells were treated with anti-Nestin (NBP1-92717; Novus Biologicals, Centennial, CO, USA), anti-neuronal nuclei (NeuN; ab177487; Abcam), anti-glial fibrillary acidic protein (GFAP; MAB360; EMD Millipore, Burlington, MA, USA), ionized calcium-binding adapter molecule1 (Iba1; ab178846; Abcam), anti-calbindin (ab1778; Abcam), calretinin (MAB1568; EMD Millipore), anti-insulin gene enhancer protein (islet1; ab20670; Abcam), and class III β-tubulin (Tuj1; 801201; BioLegend) and incubated overnight at 4 °C. One day after primary antibody treatment, cells were washed with 1 × PBS, incubated with Alexa Fluor 488 and 555, and goat anti-mouse or -rabbit conjugated secondary antibodies (Invitrogen) for 1.5 h at RT. Then, cells were washed three times with 1× PBS for 5 min each and mounted with DAPI-containing VECTASHIELD anti-fade mounting medium (H-1200; Vector Laboratories, Burlingame, CA, USA). Single-cavity microscope slides (76 × 26 mm2 with ground edges; 1042001; Heinz Herenz, Hamburg, Germany) were used to mount vestibular ganglion organoids. Cells were observed with a confocal microscope (FV31-S; Olympus, Tokyo, Japan) to obtain representative images. The Nestin- and NeuN-positive cells were manually counted, and Nestin lengths were measured using ImageJ. The length of Nestin-expressing cells was obtained by measuring from the soma to the edge of Nestin-positive cells. Cell population analysis was carried out in more than four randomly selected regions of interest from three batches of samples.
Electrophysiology
Whole-cell currents were recorded from cultured vestibular ganglion cells differentiated for up to 21 days (D21). All experiments were performed at RT using an EPC-8 amplifier (HEKA, Lambrecht, Germany). Electrodes (3–5 MΩ) were filled with a solution containing Cs-methanesulfonate (140 mM; Sigma–Aldrich), EGTA (0.1 mM; Merck, Darmstadt, Germany), HEPES (20 mM; Gibco), Mg-ATP (1 mM; Sigma–Aldrich), Na2GTP (1 mM; Merck), sucrose (10 mM; Sigma–Aldrich), and TEA-Cl (10 mM; Tocris Bioscience, Toronto, Canada). The internal solution was titrated with CsOH (Merck). The data were filtered at 5 kHz (EPC-8; HEKA), digitized at 10 kHz, and stored in the computer via WinWCP software (ver. 5.7.0; University of Strathclyde, Glasgow, UK) for offline analysis. The stored data were analyzed using Clampfit 11.2 (Molecular Devices, San Jose, CA, USA).
More detailed information related to organoids’ whole cell patch clamp technique was described in results 2.7.
Statistical analysis
Data were analyzed using GraphPad Prism version 10.1.2 (GraphPad Software Inc., San Diego, CA, USA) and one-way ANOVA with Tukey’s post hoc test used for multiple comparisons unless stated otherwise. P-values < 0.05 were considered significant.