Characterization of BP preparations
SEM analysis revealed prepared BP with clear edges and sizes ranging from 100–250 nm. And the samples displayed clear lattice fringes with the same d-spacing of 2.75 Å as the BP crystal (111) (Fig. 2A). As shown in Fig. 2B, BP samples had an average thickness of 5 nm when measured using AFM. These results indicated that we prepared BP with favorable crystallinity and size. Figure 2C shows the XRD patterns for these BP preparations, with diffraction peaks in accordance with standard BP crystal Bragg diffraction peaks (JCPDS Card No.: 74-1878), confirming purity. The Raman spectrum of BP (Fig. 2D) also shows three expected characteristic peaks (\({\text{A}}_{\text{g}}^{1}\), B2g and \({\text{A}}_{\text{g}}^{2}\)).
A–D SEM image, AFM image, XRD spectrum and Raman spectrum of exfoliated BP, respectively. HRTEM images of BP are inset in (A). E STEM image of BP-PEG. F–H P, O and N element mapping from (E), respectively. I FTIR spectra of BP, BA, and BP-BA. J, K DLS and zeta potential curves of BP and BP-BA. L In vitro release profiles. Data are presented as means ± SD (n = 6). **p < 0.01 vs the BP-BA group
We modified the BP surface with PEG-NH2 and then loaded BA through electrostatic reactions. The successful preparation of BP-BA was then demonstrated by a series of characterizations. Firstly, the element distribution in BP-PEG was evaluated via STEM-based EDS mapping to investigate the success of synthesis (Fig. 2E–H). The STEM bright-field images were surrounded by a darker area, confirming the successful preparation of BP-PEG, which had overlapping N elements. Then, the FTIR spectra of BP, BP-PEG, BA, and BP-BA were shown in Fig. 2I and additional file 1. The BP-BA peak positions were consistent with those of BP (POx, 1103 cm−1), BA, confirming that the BP-BA was successfully prepared. We performed 1H NMR analysis for further validation (Additional file 2). 1H NMR analysis revealed that the interactions in BP-BA were mainly through π-π stacking and electrostatic interactions between BA and BP-PEG. DLS analysis showed that BP had an average size of 211.5 nm with a PDI of 0.175. PEG modification reduced size and PDI to 190.3 nm and 0.119, respectively, while BA incorporation increased BP-BA size to 261.7 nm and PDI to 0.176 (Fig. 2J, Additional file 3). The zeta potential value was BP at − 28.6 mV, BP-PEG at − 25.2 mV, and BP-BA at − 14.1 mV (Fig. 2K, Additional file 3). There is a significant reduction in the absolute zeta value of the resultant material due to the addition of BA to BP. The larger particle size of BP-BA than pure BP observed by DLS was therefore attributed to the agglomeration of BP-BA particles with low absolute zeta values. In general, the fabricated BP-BA was subsequently loaded in MNs within one day. we tested the storage stability of the BP-BA and measured the hydrated particle size and PDI in water on days 0, 3 and 5, respectively. As shown in Additional file 4, the particle size of BP-BA after 5 days increased only 14 nm and the PDI increased ~ 0.04 compared to day 0, indicating that BP-BA has good short-term stability.
In vitro release assay demonstrated that 635 nm irradiation (+ 635 nm) significantly promoted BA release from BP-BA (Fig. 2L), with a 31.40 and 26.37% increase in release within 24 h compared to BA alone and BP-BA without 635 nm laser (BP-BA), respectively. This confirms that light irradiation enhance the drug release [42, 43].
Characterization of photo-thermal performance
We next examined the photo-thermal absorption properties of BP and BP-BA. BP exhibited decreasing absorbance with increasing wavelength (Fig. 3A), and the addition of BA led to a reduction in absorbance (Fig. 3B). This reduction was due to the loss of BP during the preparation of BP-BA and the lower dispersibility of BP-BA compared to pure BP.
To evaluate the repeatability of the thermal behavior induced by 635 nm laser irradiation, BP and BP-BA formulations underwent five consecutive on/off cycles of 5 min laser exposure followed by 5 min of natural cooling (Fig. 3C, D). Both formulations showed an increase in temperature with time during irradiation, the temperature of BP (Fig. 3C) and BP-BA (Fig. 3D) increased from 27.92 °C and 27.88 °C to 30.57 °C and 30.03 °C after the first irradiation cycle, respectively. Based on the cooling curve yielded after the first PT cycle, the PT conversion efficiency values were calculated to be 23.1 and 21.7% for BP (Fig. 3E) and BP-BA (Fig. 3F), respectively, indicating that the addition of BA slightly reduced the PT conversion efficiency of pure BP.
We further verified whether these preparations possessed heat shielding properties by monitoring the temperature at different locations with 1 mL of large volume of different concentrations of BP or BP-BA subjected to light irradiation (0.5 W/cm2, 635 nm) for 3 min. As shown in additional file 5, the temperature of high concentration (50 μg/mL) of BP or BP-BA reached a maximum temperature of 32 ℃ during this time period, and the temperature became lower as the detection moved towards the bottom, indicating that the preparations do not produce heat damage to deep skin tissues and have excellent heat shielding properties. Additionally, there was no overheating reaction to the scalp even with large volumes of the preparation solution administered with light irradiation.
In vitro PT performance of BP was also evaluated by irradiating samples of PBS, BP, and BP-BA with a laser irradiation (0.5 W/cm2, 635 nm) for 3 min. Temperature changes were monitored every 30 s usining an IR camera. The results revealed significant PT performance in a time- and concentration-dependent manner (Additional file 6), with both BP and BP-BA showing a rapid and marked temperature increase to approximately 41 °C after just 3 min of 635 nm irradiation. In contrast, the temperature of PBS sample increased only 2 °C after irradiation.
Assessment of in vitro anti-AGA efficacy
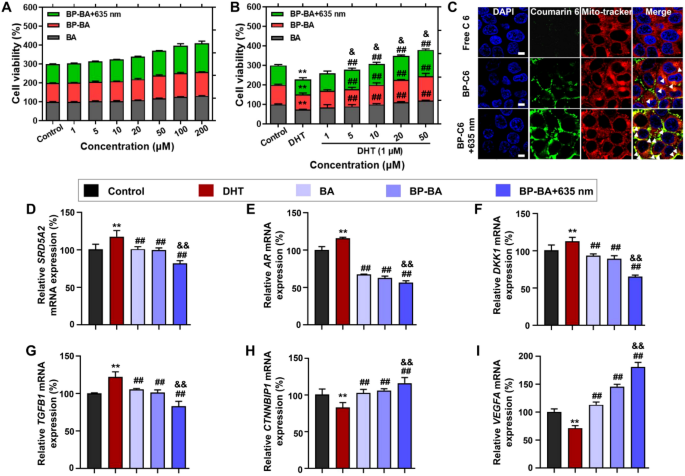
The preparations of BA, BP, BP + 635 nm (without BA), BP-BA (without 635 nm laser irradiation), or BP-BA + 635 nm did not show significant cytotoxicity in vitro, and BA or 635 nm irradiation even promoted the proliferation of hDPCs (Fig. 4A and additional file 7). Subsequently, we observed the ability of these preparations to protect hDPs against the DHT induction in vitro [44]. Compared to the control group (the untreated cells), the treatment with 1 μM of DHT reduced the cell viability of hDPCs to approximately 72% (Fig. 4B). When co-incubated with varying concentrations of BA, BP-BA, or BP-BA + 635 nm (containing BA of 1, 5, 10, 20 or 50 μM) for 24 h, there was a dose-dependent increase in cell viability, indicating a protective effect against DHT-induced cell damage. We additionally employed CLSM to visualize the cellular uptake and localization of C6-labeled preparations in hDPCs. Compared to the free C6 group, the fluorescence intensity in cells treated with C6-BP without 635 nm irradiation and C6-BP with 635 nm irradiation (C6-BP + 635 nm) was 2.6 and 4.6 times higher, respectively (Additional file 7). This indicated that BP enhanced C6 cellular uptake and 635 nm irradiation further boosted the uptake. C6 was localized in the mitochondria of the cells (Fig. 4C).
In vitro biocompatibility analysis and effects on cellular levels in DHT-induced hDPCs. A The viability of hDPCs treated with BA, BP-BA, and BP-BA + 635 nm. B The indicated treatments were evaluated for their ability to prevent DHT-induced cytotoxicity. C Fluorescence co-localization images in mitochondria of different C6-labeled groups, as imaged by CLSM. Scale bar: 5 μm. D–I The indicated groups regulated mRNA expression levels of DHT-induced hDPCs. They inhibited the mRNA expression of negative genes of SRD5A2 (D), AR (E), DKK1 (F) and TGFB1 (G), and activated the mRNA expression of positive genes of CTNNBIP1 (H), and VEGFA (I) in DHT-induced hDPCs. Data are means ± SD (n = 6). **p < 0.01 vs the Control group. ##p < 0.01 vs the DHT group. &p < 0.05 and &&p < 0.01 vs the BP-BA group
To elucidate the molecular mechanisms of these preparations for anti-AGA, we conducted RT-PCR tests to measure the expression of mRNA levels of various genes in an in vitro DHT-induced AGA cell model. According to Fig. 4D–I, after treatment with DHT, hDPCs exhibited increased mRNA expression of negative regulators such as SRD5A2, AR, DKK1, and TGFB1, and decreased expression of positive factors CTNNBIP1 and VEGFA compared to the control group (Fig. 4D–G, and H–I). This suggests that DHT induction has an impact on gene expression, leading to a dysregulation of factors that play a crucial role in hair growth [45]. As expected, treatment with the various preparations significantly reduced the mRNA expression levels of SRD5A2, AR, DKK1, and TGFB1, while enhancing those of CTNNBIP1 and VEGFA compared to the DHT-treated group. The abnormal mRNA expressions relative with AGA were normalized by BA formulations, and the amelioration effects was in turn of BP-BA + 635 nm > BP-BA > BA. Interestingly, VEGFA expression significantly increased following 635 nm irradiation (BP-BA + 635 nm), to 2.5 times that of the model group. This result highlights that 635 nm irradiation had a significant promoting effect on angiogenesis [46].The mild thermal effects of BP upon 635 nm irradiation presented the promotion effects in the cellular level.
Anti-AGA efficacy in isolated HF organ culture
Mice hair growth ex vivo correlates with hair growth in vivo, although HFs typically begin to regress and cease growth after a few days ex vivo without treatment. We evaluated the effects of various formulations on HF activity in terms of the elongation and maintenance of the growth phase of isolated HFs. Additional file 8 shows the mice vibrissa HF organ culture with different formulations. After a 12 day treatment period, 8 μM of DHT reduced the hair shaft length of vibrissae by about 33% compared to the control treatment. However, subsequent treatments with BA, BP-BA, and BP-BA + 635 nm significantly increased hair shaft length by approximatel 14, 19 and 24%, compared to the DHT group. These results confirm that BA or the mild thermal effect from 635 nm irradiation enhance the HF growth ex vivo, with the combined treatment showing enhanced efficacy in tissue level.
Characterization of BP-BA@MNs
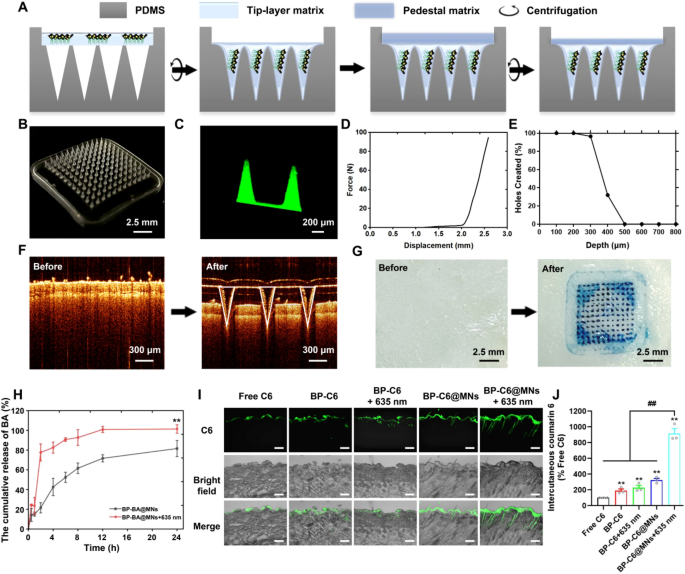
Show Fig. 5A shows the fabrication process of BP-BA@MNs. Brifely, the HA solution containing BP-BA was filled into the MN mold followed by centrifugation to form the MN tips. Subsequently, pure HA solution was added as the backing material, and the fully dried BP-BA@MNs were removed from the mold. BP-BA@MNs were visualized with digital optical microscopy and CLSM. The MNs were arranged in an 12 × 12 array with a length of 800 μm from pedestal to tip on a 10 × 10 mm2 patch (Fig. 5B). The MN tips displayed a uniform distribution of BP-C6 (C6 mimicked BA for visualization), indicating that BA was evenly distributed in the tip (Fig. 5C). As determined by the texture analyzer, the BP-BA@MNs patch had a mechanical strength of 98.06 ± 0.68 N/patch (Fig. 5D), sufficient to penetrate skin without breaking [47]. Subsequently, to measure the insertion depth of the MNs, we used Parafilm M® as a skin mimic The higher puncture pore ratio allowed the BP-BA@MNs to penetrate Parafilm M® up to the fourth layer (Fig. 5E), indicating an insertion depth of about 400 μm. OCT confirmed that the MNs reached a maximum depth of about 300 µm in isolated rat skin, with no breakage observed during insertion (Fig. 5F). The penetration depth of the BP-BA@MNs was less than 400 μm, this was mainly because of the elasticity of the skin which served as a barrier to the BP-BA@MNs. Methylene blue staining pre- and post-insertion the skin illustrated the MNs’ effective porogenic integrity and insertion efficacy, highlighting their potential for transdermal drug delivery (Fig. 5G).
Fabrication and characterization of BP-BA@MNs. A schematic fabrication process of BP-BA@MNs. Photographs of BP-BA@MNs array and needle taken with (B) digital microscopy and (C) confocal laser scanning microscopy. BP-BA@MNs were characterized by (D) mechanical strength and (E) insert depth. F Images of the optical coherence tomography (OCT) of isolated rat skin before and after BP-BA@MNs application. G Photographs of SD rat skin before and after insertion of methylene blue (MB)-BP-BA@MNs. H In vitro release profiles. Data are presented as means ± SD (n = 6). **p < 0.01 vs the BP-BA@MNs group. I CLSM images of C6 permeation through the rat skin after 4 h treatment with different formulations. Scale bar: 200 μm. J Quantitative analysis of intercutaneous C6 fluorescence intensity. Data are means ± SD (n = 3). **p < 0.01 vs the Free C6 group. ##p < 0.01 vs the BP-BA@MNs + 635 nm group
The drug loading of BA in BP-BA@MNs determined with HPLC was 11.68 ± 1.93 μg per patch. The cumulative release rate within 24 h of BA from MNs with or without 635 nm irradiation was 100.31 ± 3.33% and 76.18 ± 8.77%, respectively (Fig. 5H), demonstrating that 635 nm laser irradiation significantly enhances the release rate by 24.05%, consistent with our previous in vitro release findings of BP-BA + 635 nm.
The storage stability of BP-BA@MNs was evaluated in terms of the mechanical strength, insertion depth, and BA content of BP-BA@MNs at 0, 7, and 14 days, respectively (additional file 9). The results showed that compared with day 0, the mechanical strength of BP-BA@MNs remained above 90 N after 14 days, and there was no significant changes in the insertion depth and the content of BA in BP-BA@MNs. Therefore, BP-BA@MNs have good storage stability. However, the long-storage test of BP-BA@MN is being investigated.
Measurement of HF permeability in vivo
The skin is primarily composed of three layers: epidermis, dermis, and subcutaneous tissue. The epidermal layer is further divided into the stratum corneum (SC) and the viable epidermis. The thickness of the SC, viable epidermis, and dermis is 10–20 μm, 100–150 μm, and 3–5 mm, respectively [48]. HFs, as skin appendages, are anchored in the skin of dermis, targeted HF delivery system is anticipated to deliver drugs to dermis.
To assess the efficiency of the preparations in targeting HFs and transdermal penetration in vivo, we topically applied C6, BP-C6, BP-C6 + 635 nm, BP-C6@MNs or BP-C6@MNs + 635 nm on mice. CLSM was used to analyze cryosections of dorsal skin following these applications. As shown in Fig. 5I and J, after 4 h of treatments, C6 from the C6 suspension predominantly accumulated in the SC, specifically within the depth of 10–20 μm. While C6 from BP-C6 predominantly concentrated in the SC and slightly permeated into the viable epidermis, reaching the depth of ~ 40 μm. This indicated that C6 was difficult to effectively penetrate the skin when a single loading system was utilized [49]. After irradiated with 635 nm laser, the C6 permeated into deeper viable epidermis at the depth of ~ 100 μm. The irradiation with 635 nm laser enhanced the drug penetration due to the PT effect. The BP-C6@MNs delivered C6 at the depth of ~ 180 μm. Furthermore, BP-C6@MNs under 635 nm irradiation (BP-C6@MNs + 635 nm) resulted in a significant enhancement in permeation depth of C6, reaching approximately 350 μm in the dermis, and the C6 fluorescence was observed along the HF. This suggested that MNs are highly effective in targeted drug delivery to follicular structures. Besides, it has been reported that HA as the MN materials exhibits a better affinity for HF-related structures [50]. Quantitative fluorescence analysis revealed that BP-C6@MNs + 635 nm was 4.04-fold and 2.84-fold to BP-C6 + 635 nm and to BP-C6@MNs, respectively (Fig. 5J). These results provide an evidence that the combination of PT and MNs is a highly effective method for delivering drugs to the skin [51]. PT-assisted drug delivery could be a promising approach for treating a wide range of skin conditions, offering an effective way to deliver drugs to the skin and accumulate in the HF site.
Hair growth efficiency in the AGA model
The PT efficacy of BP-BA or BP-BA@MNs was evaluated in vivo. Using a thermal camera, we monitored the temperature changes (ΔT) of animal skin under 635 nm laser irradiation at a power density of 0.5 W/cm2 for 3 min (Additional file 10). The animals treated with PBS showed a minimal ΔT of 1.7 °C. In contrast, those treated with BP-BA or BP-BA@MNs exhibited a much higher change in temperature, up to 14.4 °C and 14.3 °C, respectively. Consequently, mice subjected to these treatments attained final dorsal temperatures of 41.3 °C and 41.9 °C, aligning with with the requirements for in vivo mild photothermal therapy [52]. The PT efficacy was consistent with the results of in vitro experiments.
To further investigate the effects of these preparations on hair growth, an AGA model was induced by testosterone. Additional file 11 shows a schematic diagram of the AGA modeling and treatment. Throughout the 15 days’ treatment, formulations containing MNs were applied every three days, whereas other treatments were applied daily. Figure 6A and Additional file 12 show the representative and all (n = 6) dorsal skin photographs of the hair re-growth process from day 0 to day 15 after treatment. Hair growth was visually documented and quantitatively scored throughout this period. Notably, the BP-BA@MNs + 635 nm treatment had significantly higher hair growth scores compared to the other groups, although slightly lower than the control and MXD group (Fig. 6B). After 15 days’ treatment, hair coverage area demonstrated that BP-BA@MNs + 635 nm treatment was the most effective in promoting hair growth, achieving 93.63% hair regrowth (Fig. 6C). In comparison, the BP-BA + 635 nm group and BP-BA@MNs group only resulted in 56.98 and 57.77% of hair regeneration, respectively. The average hair length of the mice in the BP-BA@MNs + 635 nm group was longer than that the other group (Fig. 6D). Together, the enhanced efficacy of the BP-BA@MNs + 635 nm highlights the synergistic effect of MNs and PT in promoting hair regeneration in an AGA model, even with reduced frequency of application.
Evaluation of the pharmacodynamics in vivo. A Representative photographs of hair growth on the dorsal skin on day 0, 3, 6, 9, 12, and 15. B Quantitative distributions of hair growth score of the dorsal skin of mouse treated with different formulations during days 0–15. The corresponding skin color grayscale ratio (C) and hair length (D) on day 15 post-treatment. Data are means ± SD (n = 6). H&E staining of hair follicle regeneration on the longitudinal (E) and transverse (G) mouse dorsal skin. F Quantitative analysis of skin thickness on day 15 post-treatment. Quantitative analysis of (H) the number of hair follicle and (I) the ratio of terminal hair/vellus hair. Data are means ± SD (n = 3). **p < 0.01 vs the Control group. ##p < 0.01 vs the Model group. &&p < 0.01 vs the BP-BA@MNs group
H&E staining for hair regrowth evaluation
Histological analysis using H&E staining of dorsal skin after 15 days of treatment confirmed the promotion of hair regrowth (Fig. 6E–I). AGA is characterized with thinned skin, smaller dermal papillae, and fewer HF due to inadequate blood supply. This pathology often leads to the upward migration and gradual miniaturization of HFs, resulting in the replacement of terminal hairs with vellus hairs [53]. As shown in Fig. 6E, the model group exhibited significant skin thinning, minimal dermal papillae, and miniaturized HFs. In contrast, mice treated with different formulations displayed increased skin thickness and enlarged hair bulbs, characteristics associated with the mid-late anagen phase of hair growth, clearly delineating the differences in hair density and morphology between treated groups and the model group (Fig. 6E). Notably, the testosterone-induced model group showed a 34.43% reduction in skin thickness compared to the control group (Fig. 6F). At the same time, the mice treated with blank MNs, BP-BA@MNs, and BP-BA@MNs + 635 nm showed no irreversible skin damage on the 15th day post-treatment, indicating that transdermal administration of MNs is safe.
HFs pass through three distinct stages during their life cycle: rest (telogen), growth (anagen) and regression (catagen) [54]. During the anagen phase, the number and size of HF increase. Normally, the scalp produces more terminal hairs than vellus hairs. However, in our AGA model induced by testosterone, both the volume and number of HF decreased, altering the ratio of terminal to vellus hairs with an increase in vellus hairs. Concurrently, the hair shaft underwent a reduction in both the number and the diameter (Fig. 6G). Treatment with various formulations significantly increased the number of HFs and improved the ratio of terminal to vellus hairs, demonstrating the effectiveness of the treatments in reversing testosterone-induced changes (Fig. 6H and I). The ratio of terminal to vellus hairs for the different groups was as follows: MXD group (49.10 ± 15.78%), BP-BA + 635 nm group (46.22 ± 5.82%), BP-BA@MNs group (43.68 ± 11.38%), and BP-BA@MNs + 635 nm group (51.46 ± 8.27%).
Immunofluorescence staining for hair regrowth evaluation
The Wnt/β-catenin signaling pathway plays a significant role in the hair cycle, acting as the key driving force in the transition from telogen to anagen [55]. Abnormal regulation of this pathway can lead to hair growth disorders, which affects the size and shape of HF. The expression of β-catenin was analyzed via immunofluorescence staining. In the testosterone-induced model group, β-catenin expression significantly decreased and was primarily localized in the upper region of the HFs. Conversely, treatment with BP-BA@MNs + 635 nm markedly increased β-catenin expression, concentrating it in the lower part of the HF, within the outer and inner root sheath (Fig. 7A and B). This suggests that the treatment with BP-BA@MNs + 635 nm facilitates the transition of HF growth cycle. Additionally, areas treated with the preparations showed higher levels of Ki67, a marker of cell proliferation, consistent with anagen phase induction Fig. 7C and D). The expression levels of Ki67 in the MXD group, BP-BA + 635 nm group, BP-BA@MNs group, and BP-BA@MNs + 635 nm group were 3.20, 2.70, 2.59, and 3.48-fold to that of the model group, respectively.
A Representative images of β-catenin immunofluorescence staining on depilated skin on day 15 post-treatment. Scale bar: 100 μm. Images represent at the HF site. B Quantification of the relative expression of β-catenin on the mice dorsal skin treated with different groups. Data are means ± SD (n = 3). C Representative images of immunofluorescence staining of Ki67 and CD31 on depilated skin on day 15 to investigate the expression of proliferation markers and perifollicular angiogenesis. Scale bar: 50 μm. Images represent at the HF site. Quantification of the relative expression of (D) ki67 and (E) CD31 on the mice dorsal skin treated with different formulations. Data are means ± SD (n = 3).**p < 0.01 vs the Control group. ##p < 0.01 vs the Model group. &&p < 0.01 vs the BP-BA + 635 nm group or the BP-BA@MNs group
Additionally, the angiogenesis of capillaries and perifollicular vessels were also examined. Immunofluorescence staining of CD31 in mice dorsal skin revealed increased blood vessel formation in alopecia areas treated with the indicated preparations compared to the model group (Fig. 7C). This increase could be attributed to: 1) BA has been reported to have a beneficial effect on promoting angiogenesis [56]; 2) 635 nm irradiation promotes angiogenesis by increasing the permeability of the perivascular space, thereby increasing blood flow [57]; 3) MNs stimulate angiogenesis through mechanical stimulation [35]. Remarkably, CD31 expression in BP-BA@MNs + 635 nm group was four times higher than that of the model group, comparable to that daily topical application of commercial MXD group, despite the fact that the BP-BA@MNs + 635 nm was administered every three days during the treatment period for only five applications (Fig. 7E). Thus, the molecular mechanisms underlying these observed effects will be elucidated in the further experiments.
Determination of HF-related mRNA expression in AGA
Hair regrowth is highly regulated by various factors involved in the HF cycle, whose inhibition or activation affects hair growth. Based on in vitro cellular experiments, we have confirmed that both BA and 635 nm irradiation effectively regulate hair growth-related factors. To elucidate the molecule mechanisms by which the indicated preparation groups restored or promoted hair growth, we analyzed the changes in the same signaling molecules as the in vivo experiments (Fig. 8). AGA is closely associated with the expression of Srd5a2, an enzyme critical for converting testosterone into DHT, a potent androgen with a very high affinity tor Ar [58, 59]. Elevated levels of Srd5a2 and Ar are known primary drivers of AGA. In our study, the model group significantly increased Srd5a2 and Ar mRNA expressions by 2.02-fold and 2.46-fold, respectively. In comparison to the model group, the MXD group, the BP-BA + 635 nm group, the BP-BA@MNs group, and the BP-BA@MNs + 635 nm group markedly reduced mRNA expressions of Ar by 10.16, 39.15, 40.61, and 47.69%, respectively and Srd5a2 by 42.25, 33.16, 33.62, and 38.21%, respectively (Fig. 8A and B). More importantly, the BP-BA@MNs + 635 nm treatment reduced Ar mRNA expression more than 1.71-fold compared to the MXD group. Molecular docking simulation supported this finding by displaying BA’s strong binding affinity to Ar and Srd5a2, providing a theoretical basis for its efficacy in targeting the two major pathological factors of AGA (Additional file 13).
The mRNA expression levels in the dorsal skin of the AGA mice treated with different formulations. They inhibited the mRNA expression of negative genes of Srd5a2 (A), Ar (B), Dkk1 (C) and Tgfb1 (D), and activated the mRNA expression of positive genes of Ctnnb1 (E), and Vegfa (F). Data are means ± SD (n = 6). **p < 0.01 vs the Control group. ##p < 0.01 vs the Model group. &&p < 0.01 vs the BP-BA + 635 nm group or the BP-BA@MNs group
As mentioned previously, β-catenin promotes the induction and duration of the HF anagen phase, while Dkk1, a potent antagonist of the Wnt/β-catenin signaling pathway, drives the catagen phase and apoptotic cell death in HFs [60, 61]. Similarly, Tgfb1 is a recognized promoter of the catagen phase [62]. In this context, our results showed that testosterone induction significantly decreased Ctnnb1 expression while increasing Dkk1 and Tgfb1 expressions (Fig. 8C–E). The treatment with BP-BA@MNs + 635 nm significantly reversed these effects, elevating Ctnnb1 expression by 78.52% and reducing Dkk1 and Tgfb1 expressions by 71.47% and 65.49%, respectively, outcomes that not only improved upon other treatment groups but were also comparable to the MXD group.
VEGF, a biomarker of angiogenesis and a key growth factor for hair growth, was shown to have a good pro-angiogenic effect from the immunofluorescence results in the indicated preparation groups. Here we further evaluated the effect of different formulations on the expression of Vegfa mRNA at the molecular level (Fig. 8F). The results showed that Vegfa mRNA was significantly more expressed in the alopecia areas treated with the above indicated preparation groups compared to the model group, consistent with CD31 staining results. Remarkably, even a single mode of therapy in the preparation groups promoted a high level of Vegfa mRNA expression, thus supporting our interpretation of high CD31 expression.
In summary, testosterone induction disrupts normal HF growth by altering the expression of crucial genes. Our BP-BA@MNs + 635 nm treatment effectively counteracted these changes, offering a targeted therapeutic approach to AGA by normalizing gene expression related to HF cycling and growth.
In vivo biocompatibility evaluation
After a 15-day treatment, we collected blood samples from the animals and analyzed them for hematological parameters and serum enzyme levels. The results were documented in Additional file 14. There were no significant differences across the various groups, indicating that the treatments did not cause significant hepatic and renal toxicity. Further analysis of major organs using H&E staining revealed no significant tissue damage, and there was also no observed weight loss in the treated mice. These findings affirm that the treatments possess good histocompatibility, suggesting their safety for prolonged use.