Characterization of the micelles
Dual-targeted and dual-response intelligent micelles were prepared to exploit the inflammatory microenvironment of RA. The fabrication process is shown in Scheme 1. The transmission electron microscopy revealed that TK-HA-Cel@Ms had a homogeneous, spherical morphology at the nanoscale, with smooth and rounded surfaces, uniform sizes, and diameters of approximately 40 nm (Fig. 1A), which corresponded well with Nano Series Zen 4003 Zetasizer measurements (Fig. 1B) of 46.63 ± 1.82 nm and zeta potential of − 4.70 ± 0.36 mV (Fig. 1C).
Characterization of micelles. (A) TEM image of TK-HA-Cel@Ms, scale bar = 50 nm; (B) Particle size distribution of TK-HA-Cel@Ms; (C) Zeta potential distribution of TK-HA-Cel@Ms; (D) Quantitative analysis of particle size of micelles; (E) Quantitative analysis of zeta potential of the varying micelles; (F) Quantitative analysis of PDI value of the varying micelles; (G) Quantitative analysis of encapsulation efficiency of the varying micelles; (H) Release rate of Cel from the varying formulations; (I) Hemolysis of induction of TK-HA-Cel@Ms, where “-” and “+” represented negative control and positive control; (J–L) Changes in PDI (J), encapsulation efficiency (K), and particle size (L) of TK-HA-Cel@Ms were quantitatively analyzed after 28 d of placement at 4 °C or RT. Data are presented as the mean ± SD (n = 3). a, Blank@Ms; b, Cel@Ms; c, HA-Cel@Ms; d, TK-HA-Cel@Ms; e, TK-HA-Cel@Ms + H2O2; f, TK-HA-Cel@Ms + H2O2 + HCl
Moreover, the particle size, zeta potential, and PDI values of the micelles are shown in Fig. 1D-F. All the micellar formulations had a particle size of less than 50 nm, a weakly negative potential, and a narrow polydispersity coefficient. Moreover, the particle size of TK-HA-Cel@Ms changed from 46.63 ± 1.82 nm to 40.24 ± 1.97 nm in the presence of H2O2, suggesting that it had a size-shrinkable property, which suggests that the TK bond is cleaved in ROS-rich environment, shedding the hydration layer of PEG5000 and restoring its targeting effects. PEG5000, as a longer linear polymer, has a diameter between 31 nm and 40 nm. And when PEG5000 was modified to the surface of the micelles, the particle size of the micelles only increased by about 10 nm, which indicated that PEG5000 formed a shell on the surface of the micelles, which might wrap the micelles, and this shell might not be monolayer, but had a certain thickness, which made the total particle size of the micelles increase. The particle size of TK-HA-Cel@Ms increased to 108.05 ± 5.50 nm in the presence of both H2O2 and HCl (pH = 5.5), indicating that the acid-sensitive material mPEG114-PDPA10 responded to the acidic environment, which led to the disassembly of the micellar structure. The CMC of TK-HA-Cel@Ms was 0.0357 mg·mL− 1, as shown in Fig. S1. In addition, the EE of each drug-loaded micelles was greater than 90%, and TK-HA-Cel@Ms had an EE of 93.83% ± 1.00% (Fig. 1G).
The stability of micelles under oxidising conditions was examined by monitoring the changes in particle size and PDI by DLS (Fig. S2). The particle size and PDI of TK-HA-Cel@Ms did not change much after 6 h in the absence of H2O2. Whereas, the particle size of TK-HA-Cel@Ms contracted by about 10 nm and then levelled off after 2 h in presence of 200 µM H2O2, and the PDI also increased significantly. This indicated that under the action of H2O2, the TK-sensitive bonds were broken, the PEG5000 layer on the micelle surface were shed off, and the particle size shrunk.
The drug-release behavior is a key parameter to ensure high drug activity, especially the sensitivity of drugs to inflammatory environments. Ideal nanoparticle systems can control drug release in certain stimulatory environments, such as pH or ROS, allowing for precise drug delivery [22]. Therefore, the drug release from Cel was determined using high-performance liquid chromatography (HPLC), and the drug-release profile of TK-HA-Cel@Ms in inflammatory microenvironments was simulated. Fig. 1H demonstrates the in vitro release kinetic results of TK-HA-Cel@Ms and free Cel. Free Cel was almost completely released within 48 h, while about 35% of Cel was released from TK-HA-Cel@Ms, which was significantly lower than that of free Cel. This indicated that TK-HA-Cel@Ms had significant retardation because the outermost layer of the micelles, PEG5000, formed a hydration layer, thus increasing the stability of the micelles and delaying the release of Cel. In contrast, TK-HA-Cel@Ms released more than 40% of the Cel in PBS (pH 7.4) containing 200 µM H2O2. It was probably due to the H2O2-induced breakage of the TK bond, which facilitated the release of Cel afterward. The release of TK-HA-Cel@Ms was significantly accelerated in PBS (pH 5.5) containing 200 µM H2O2, with more than 60% of Cel released for 48 h. The rapid release of Cel was attributed to the breakage of the TK-sensitive bond in the presence of ROS. This stripped off the hydration layer of PEG5000, which in turn led to the decomposition of the micelles under acidic conditions, thereby accelerating drug release. These results suggested that it was possible to ensure the stable delivery of TK-HA-Cel@Ms in circulation and trigger the cascade targeting of micelles at the site of inflammation for precise drug delivery, which is crucial for the effective treatment of RA.
After intravenous administration, TK-HA-Cel@Ms are exposed to blood cells and inflammation-related cells. Therefore, the hemocompatibility of TK-HA-Cel@Ms needs to be understood. We performed a hemolysis experiment. The group with various concentrations of drug-loaded micelles displayed a pale-yellow color. In contrast, the positive control group showed a bright red color (Fig. 1I). This suggested that the drug-loaded micelles exhibited good biocompatibility and could therefore be administered intravenously to some extent.
The stability of the micelles at 4 °C and RT was evaluated by measuring the particle size, PDI, and EE (Fig. 1J-L). The results showed that the EE of micelles slightly decreased and the particle size and PDI slightly increased within 28 days, but these variations were within the permissible range, indicating good stability of TK-HA-Cel@Ms.
Cellular uptake and distribution
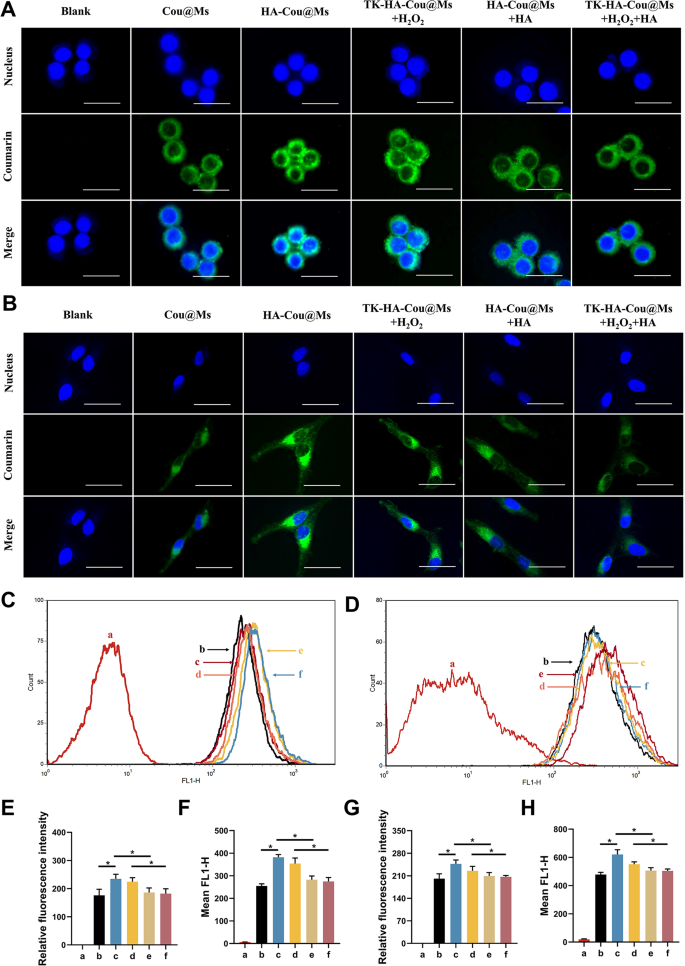
The ability of the nanoparticle systems to enter cells directly determines their therapeutic efficiency. LPS-activated RAW264.7 cells and RA-FLSs were used to assess the active targeting and cellular uptake abilities of various micelles. Confocal laser scanning imaging (Fig. 2A and B) and flow cytometry (Fig. 2C and D) showed that the green fluorescence of activated macrophages and RA-FLSs incubated with HA-Cou@Ms was the strongest. The fluorescence intensity in the TK-HA-Cou@Ms group co-incubated with H2O2 was close to that in the HA-Cou@Ms group. In addition, the uptake of HA-Cou@Ms and TK-HA-Cou@Ms co-incubated with H2O2 by activated macrophages with RA-FLSs was significantly inhibited after pretreating the cells with HA first. These results indicated that TK-HA-Cou@Ms caused the cleavage of the TK-sensitive bond by ROS, the PEG5000 on the surface of the micelles was shed, and the targeting molecule HA was exposed. HA specifically bound to the CD44 receptors on the surface of the inflammatory cells, significantly increasing the uptake and entry of the drug into the inflammatory cells through CD44-mediated endocytosis and thus displaying active targeting capability [23].
Cellular uptake after incubation with varying formulations. (A) Confocal microscope images of RAW264.7 cells treated with LPS with different formulations, scale bar, 25 μm; (B) Confocal microscope images of RA-FLSs treated with different formulations, scale bar, 25 μm; (C) Cellular uptake of different formulations to RAW264.7 cells treated with LPS; (D) Cellular uptake of different formulations to RA-FLSs; (E) Quantitative analysis of (A); (F) Quantitative analysis of (C); (G) Quantitative analysis of (B); (H) Quantitative analysis of (D). Data are presented as mean ± SD (n = 3). a, Blank; b, Cou@Ms; c, HA-Cou@Ms; d, TK-HA-Cou@Ms + H2O2; e, HA-Cou@Ms + HA; f, TK-HA-Cou@Ms + H2O2 + HA. *P < 0.05
Cell viability and apoptosis assays
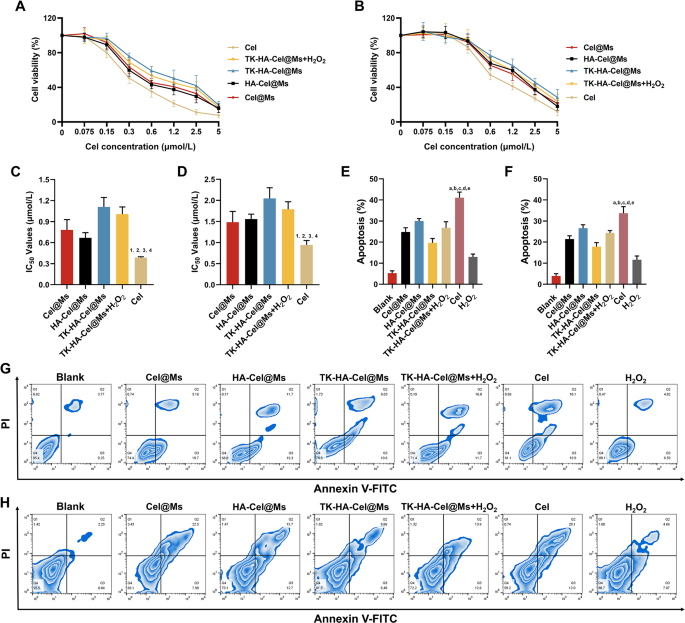
Cel has significant toxicity and side effects. Hence, the effects of free Cel and different Cel-loaded micelles on the viability of LPS-activated RAW264.7 cells and RA-FLSs in vitro were examined using the cell counting kit 8 assay. Before examining the cytotoxicity of different preparations, we first examined the effects of different concentrations of H2O2 on RAW264.7 cells and RA-FLSs cells, as shown in Fig. S3, H2O2 showed a dose-dependent cytotoxicity, but it had little effect on cell survival below 200 µM, and it was also able to effectively mimic the high ROS levels in vivo, therefore, we chose 200 µM of H2O2 for the subsequent experiments. As shown in Fig. 3A and B, all groups of drugs inhibited cell viability dose-dependently, with the free Cel showing the highest cytotoxicity and TK-HA-Cel@Ms the lowest. It was mainly because the Cel solution existed as free small molecules, which could cross the cell membrane quickly and efficiently to exert an effect. The added H2O2 increased the cytotoxicity of TK-HA-Cel@Ms, likely because the hydrated membrane formed by PEG5000 on the micelle surface effectively reduced cellular, thereby lowering cytotoxicity. Thus, it reduced normal cell damage during in vivo cycling, achieved precise delivery, and improved therapeutic efficiency. Fig. 3C and D shows the IC50 values of different preparations on LPS activation of RAW264.7 cells and RA-FLSs, more intuitively demonstrating the inhibitory effect of various formulations on cells. The ability of free Cel and different Cel-loaded micelles to induce the apoptosis of activated RAW264.7 cells and RA-FLSs was further detected using flow cytometry, and the apoptosis results were quantified (Fig. 3E-H). Cel-induced apoptosis occurred in approximately 40% of LPS-activated RAW264.7 cells and 30.0% of RA-FLSs during the 24-h test period. The micellar formulations induced lower apoptosis rates than Cel. TK-HA-Cel@Ms displayed the weakest apoptosis induction, with approximately 20% and 15% apoptosis in the aforementioned two cells, respectively, aligning with the cell viability results.
Cell viability and apoptosis after incubation with varying formulations. (A) Growth curves of varying formulations of RAW264.7 cells at varying concentrations of Cel; (B) Growth curves of varying formulations of RA-FLSs at varying concentrations of Cel; (C) Statistical analysis of IC50 values of RAW264.7 cells; (D) Statistical analysis of IC50 values of RA-FLSs; (E) The total percentages of apoptosis of RAW264.7 cells after administration with varying formulations; (F) The total percentages of apoptosis of RAW264.7 cells after administration with varying formulations; (G) The apoptosis of RAW264.7 cells after incubation with varying formulations; (H) The apoptosis of RA-FLSs after incubation with varying formulations. Data are presented as the mean ± SD (n = 3). 1, vs. Cel@Ms; 2, vs. HA-Cel@Ms; 3, vs. TK-HA-Cel@Ms; 4, vs. TK-HA-Cel@Ms + H2O2. P < 0.05. a, vs. Blank; b, vs. Cel@Ms; c, vs. HA-Cel@Ms; d, vs. TK-HA-Cel@Ms; e, vs. TK-HA-Cel@Ms + H2O2. P < 0.05
Macrophage polarization in vitro
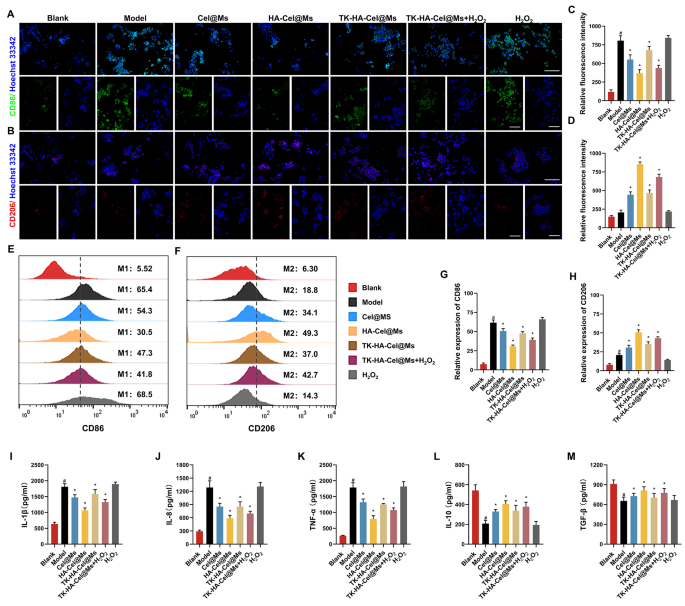
The M1/M2 transformation of macrophages is a key factor in the pathogenesis of RA. Only a few macrophages are present in the knee synovium of healthy individuals [24,25,26]. However, the intensity and filtration area of macrophage are significantly increased in the knee synovium of patients with RA [27]. Given the ability of TK-HA-Cel@Ms to target both activated macrophages and RA-FLSs, we predicted that TK-HA-Cel@Ms might be advantageous in reprogramming the phenotype of macrophages. Therefore, we first analyzed macrophage phenotypes by immunofluorescence staining of CD86 (M1 marker) and CD206 (M2 marker) and assessed whether TK-HA-Cel@Ms could effectively repolarize M1 macrophages to the M2 phenotype. As shown in Fig. 4A and B, most of the normal macrophages were of M0 phenotype with insignificant CD86 and CD206 fluorescence signals. However, the macrophages were significantly polarized to the M1 phenotype after LPS stimulation, thus showing enhanced CD86 immunity. The macrophages shifted from M1 to M2 phenotype to varying degrees after administering different drug interventions, with HA-Cel@Ms having the most pronounced effect and TK-HA-Cel@Ms co-incubated with H2O2 having the second most effective effect. The effect of TK-HA-Cel@Ms on the polarization of LPS-treated macrophages was further detected and quantified using flow cytometry. It was clearly seen that the proportion of CD86 and CD206 was about 65% and 20%, respectively, after treatment with LPS. However, a notable shift was observed in HA-Cel@Ms-treated macrophages, with a 35% decrease in the M1 phenotype marker CD86 and a 30% increase in the M2 phenotype marker CD206, indicating a significant effect on the reprogrammed macrophage polarization (Fig. 4E and F). The effect of TK-HA-Cel@Ms co-incubated with H2O2 was close to its effect, suggesting that TK-HA-Cel@Ms without the PEG5000 hydration layer in the inflammatory microenvironment significantly repolarized the M1 to M2 phenotype and reduced the M1/M2 ratio to normal levels.
Macrophage polarization and anti-inflammatory activity in vitro after incubation with varying formulations. (A) Representative fluorescence images of CD86 staining of macrophages on different formulations; (B) Representative fluorescence images of CD206 staining of macrophages on different formulations; (C–D) Quantitative analysis of fluorescence intensity in (A) and (B); (E) Flow cytometric histograms of the polarization of LPS-stimulated RAW 264.7 macrophages to the M1 phenotype; (F) Flow cytometric histograms of the polarization of LPS-stimulated RAW 264.7 macrophages to the M2 phenotype; (G–H) Analysis of relative fluorescence intensity in (E) and (F); (I–M) Levels of IL-1β, IL-8, TNF-α, IL-10 and TGF-β in cell culture supernatant. Data were presented as mean ± SD (n = 3). #, vs. Blank; *, vs. Model. P < 0.05
Anti-inflammatory activity in vitro
Inflammatory cytokines are major mediators of the inflammatory response in RA, reflecting the severity of the disease and playing an important role in the pathogenesis of immune-related RA [8, 28]. Pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-8, and tumor necrosis factor (TNF)-α secreted by M1 macrophages, can lead to increased inflammation, synoviocyte invasion, and bone destruction in RA [29]. Anti-inflammatory cytokines such as IL-10 and tumor growth factor (TGF)-β secreted by M2 macrophages are essential in inhibiting inflammation and reducing the secretion of pro-inflammatory cytokines [30]. The anti-inflammatory potential of various preparations was assessed by incubating LPS-treated RAW264.7 cells and then measuring the levels of cytokines such as IL-1β, IL-8, TNF-α, IL-10, and TGF-β. As shown in Fig. 4I-M, the levels of pro-inflammatory cytokines increased substantially, whereas the levels of anti-inflammatory cytokines decreased significantly after LPS activation. However, treatment with different preparations downregulated the levels of pro-inflammatory cytokines (IL-1β, IL-8, and TNF-α) whereas upregulated the levels of anti-inflammatory cytokines (IL-10 and TGF-β) in activated macrophages, with the most pronounced therapeutic effect of HA-Cel@Ms, followed by the second most effective effect of TK-HA-Cel@Ms co-incubated with H2O2. It suggested that the therapeutic effect of TK-HA-Cel@Ms on RA under inflammatory conditions might be attributed to the modulation of macrophage phenotypes by simultaneously enhancing anti-inflammatory macrophages and reducing pro-inflammatory macrophages.
Evaluation of proliferative, migratory and invasive activities in vitro
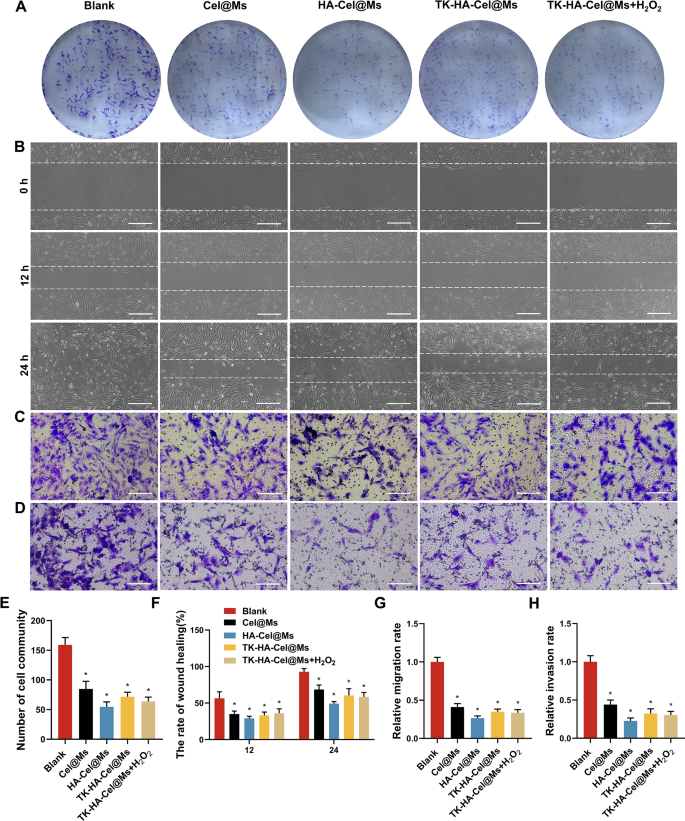
RA-FLSs present tumor-like cell growth, similar to tumor cells in terms of malignant proliferation, increased expression of intracellular anti-apoptotic genes, and subsequent erosion of cartilage, ultimately leading to joint destruction, which plays a crucial role in the pathogenesis of the aggressive phenotype of RA [31, 32]. Therefore, inhibiting the abnormal growth and proliferation of RA-FLSs is essential for RA treatment. We performed colony formation assays to investigate the effects of different micelles on RA-FLSs proliferation. The clone formation was significantly inhibited in the HA-Cel@Ms-treated group compared with the control group. The inhibitory effects on cell colony formation was significantly enhanced by TK-HA-Cel@Ms on adding H2O2 to the culture medium, which was consistent with the inhibitory effects of HA-Cel@Ms (Fig. 5A and E).
Evaluation of proliferative, migratory and invasive activities in vitro. (A) Colony formation assays of RA-FLSs on different formulations; (B) Image of wound healing on RA-FLSs in 12 h and 24 h after treatment with varying formulations, Scale bar = 50 μm; (C–D) The effects of micelles on RA-FLSs migration (C) and invasion (D) were further determined by transwell assay, Scale bar = 50 μm; (E) Number of cell community; (F) The rate of wound healing; (G–H) Relative migration and invasion rate. Data were presented as mean ± SD (n = 3). *, vs. Blank. P < 0.05
The proliferation of RA-FLSs and their subsequent migration and invasion of cartilage and bone can exacerbate joint destruction in RA. The results of the effect of each group of micellar preparations on the wound healing of RA-FLSs are shown in Fig. 5B and F. The results showed that the width of the scratches was reduced in each group 12 and 24 h after administration compared with that after 0 h, and the scratches were basically healed after 24 h in the blank group. Different micelles inhibited the healing of scratches to a certain extent compared with that in the blank group, and the HA-Cel@Ms group showed the most obvious degree of inhibition. The effects of micelles on the migration and invasion of RA-FLSs were further determined using the transwell assay. Fig. 5C, D, G, and H shows the images of the cells in each group after administering micelles and passage through the transwell. A large number of cells were seen in the blank group passing through the chamber, indicating that the RA-FLSs cells had strong migration and invasion abilities. A significant decrease in the number of cells crossing the transwell was observed in each administration group compared with the blank group, which was most obvious in the HA-Cel@Ms group. The effect of TK-HA-Cel@Ms co-incubated with H2O2 was close to it.
Targeting and circulation behavior in vivo
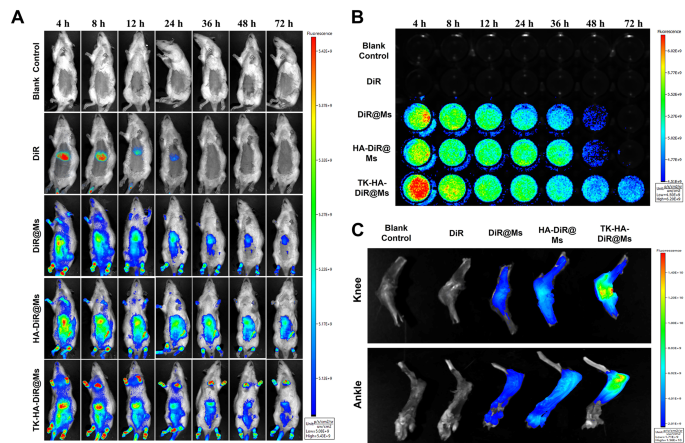
The enrichment and in vivo distribution of TK-HA-DiR@Ms of CIA model rats were observed by real-time fluorescence imaging using DiR instead of Cel as a fluorescent probe. As shown in Fig. 6A, no fluorescence signal distribution was seen in the blank group. The fluorescence of free DiR was mainly accumulated in the liver of rats, which could be explained by rapid metabolism and nonspecific targeting. However, obvious fluorescence signals were observed at the binding site in each micelle group, and these signals showed varying degrees of attenuation with time. However, the fluorescence signals of TK-HA-DiR@Ms remained concentrated at the binding site for a longer duration and were stronger in intensity.
Targeting and circulation behavior in vivo. (A) Real-time imaging observation of CIA rats treated with varying formulations in vivo; (B) Long circulation effect after intravenous administration of varying formulations in CIA rats; (C) Ex vivo imaging of the knee and ankle joints harvested from CIA rats treated with different formulations after 72 h (n = 3)
Then, the blood samples were collected from rats with CIA at appropriate time points to monitor the fluorescence intensity of the drugs in the blood and preliminarily evaluate the pharmacokinetic profile of TK-HA-DiR@Ms. No fluorescent signals were detected in the blank control and free DiR groups (Fig. 6B). In contrast, the intensity of the fluorescent signals in the blood of the rats in each micellar group gradually weakened with time. The circulation time of each agent in the blood followed the order TK-HA-DiR@Ms > HA-DiR@Ms > DiR@Ms > DiR.
The rats were executed 72 h later to collect the knee and ankle joints for fluorescence intensity analysis. As shown in Fig. 6C and D, the accumulation of each micelle at the inflamed joints significantly increased compared with free DiR, confirming that including the appropriate particle size of micelles allowed their accumulation at the lesion site using the ELVIS effect [33, 34]. In addition, the accumulation of TK-HA-DiR@Ms was most pronounced in inflamed joints. PEG5000 significantly prolonged the retention time of the micelles in the bloodstream, effectively avoiding phagocytosis by the liver and the reticuloendothelial system. Also, it concealed the targeting ligands, preventing the binding of the micelles to normal-functioning inflammatory cells, thus exerting perfect passive targeting ability. The prolonged circulation time assisted it in reaching the site of inflammation. At this site, the high levels of ROS cleaved the sensitive bonds, exposing the HA. This exposure enhanced the active targeting of the micelles, increasing the drug concentration in the joint tissues and further improving the therapeutic efficiency.
Therapeutic efficacy in vivo
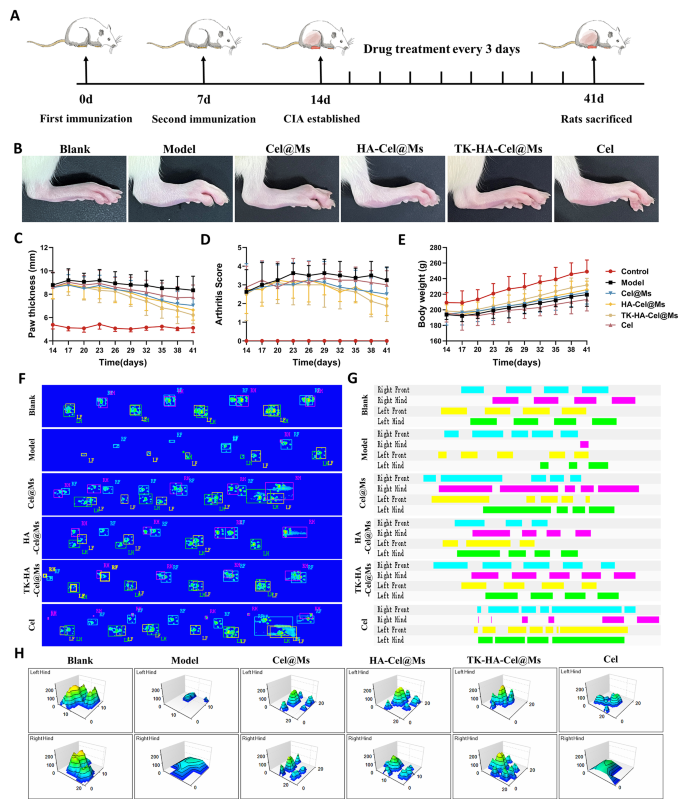
Based on the capability of TK-HA-Cel@Ms to efficiently target inflammation and slow-release drugs on demand in rats with CIA, we evaluated its combined therapeutic effects in a CIA rat model, following the protocol shown in Fig. 7A. Comparing clinical indicators, we found that the model group of rats with CIA exhibited erythema and severe deformities in multiple joints throughout the paw. In contrast, Cel@Ms, HA-Cel@Ms, and TK-HA-Cel@Ms treatments delayed the progression of RA, with TK-HA-Cel@Ms showing the best results; no significant erythema or deformities were observed in treated hind paws (Fig. 7B). In addition, TK-HA-Cel@Ms significantly reduced paw thickness and arthritis index in rats with CIA (Fig. 7C and D). The quality of life of rats with CIA was assessed by comparing the changes in body weight. Rats in the model group showed slow weight gain from disease onset to peak, whereas rats treated with TK-HA-Cel@Ms demonstrated weight gain similar to that of healthy rats. This indicated that TK-HA-Cel@Ms improved the quality of life of the rats (Fig. 7E).
Anti-arthritic efficacy in CIA rats. (A) Schematic illustration of treatment regimens; (B) Representative photographs of hindlimbs from each group; (C) Paw thickness of arthritic rats over time from each group; (D) Arthritis score of arthritic rats over time from each group; (E) Body weight of arthritic rats over time from each group; (F) Footprint-pressure thermographic map in CIA rats; (G) 2D imaging of rat plantar pressure; (H) 3D imaging of rat plantar pressure (n = 6)
We then assessed whether TK-HA-Cel@Ms could restore rats with CIA to a normal walking pattern. The fluorescence images of the rat paws touching the surface of the glass plate showed that static parameters, including mean paw pressure and mean paw area, were reduced in rats with CIA compared with normal rats. However, TK-HA-Cel@Ms significantly restored paw pressure and paw area (Fig. 7F). Also, the 2D and 3D plots revealed that the rats in the TK-HA-Cel@Ms group had significant plantar pressures and longer retention times compared with the rats in the model group, which was close to that in the blank group (Fig. 7G and H).
Further, the serum levels of IL-1β, IL-8, TNF-α, IL-10, and TGF-β were measured to verify the effect of TK-HA-Cel@Ms on the production of inflammatory cytokines, which are closely related to the development of RA. As shown in Fig. S4, TK-HA-Cel@Ms could significantly downregulate the levels of pro-inflammatory cytokines (IL-1β, IL-8, and TNF-α) in the serum of rats with CIA while up-regulating the levels of anti-inflammatory cytokines (IL-10 and TGF-β) in the serum. It was consistent with the results of the in vitro experiments.
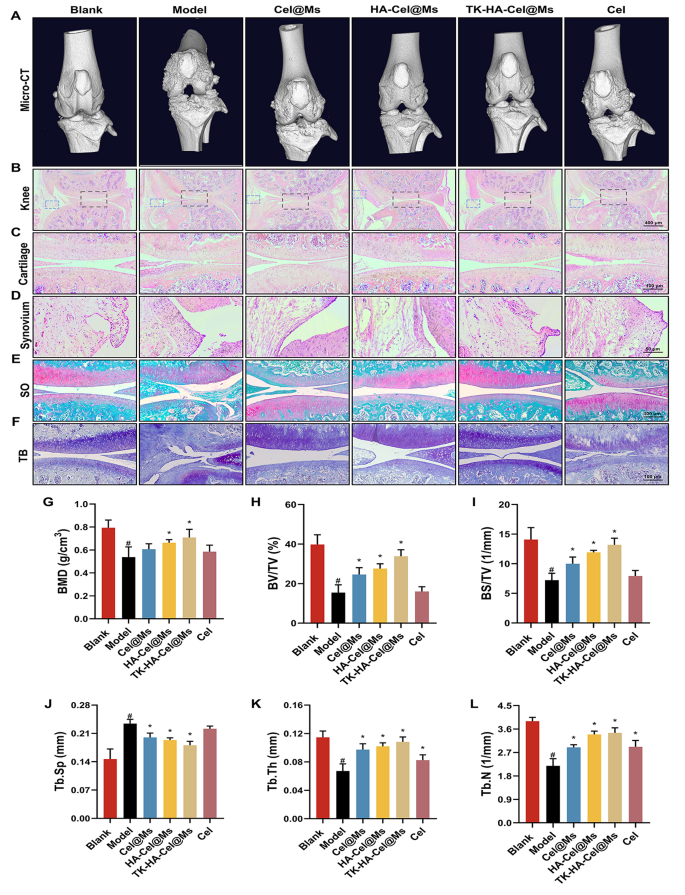
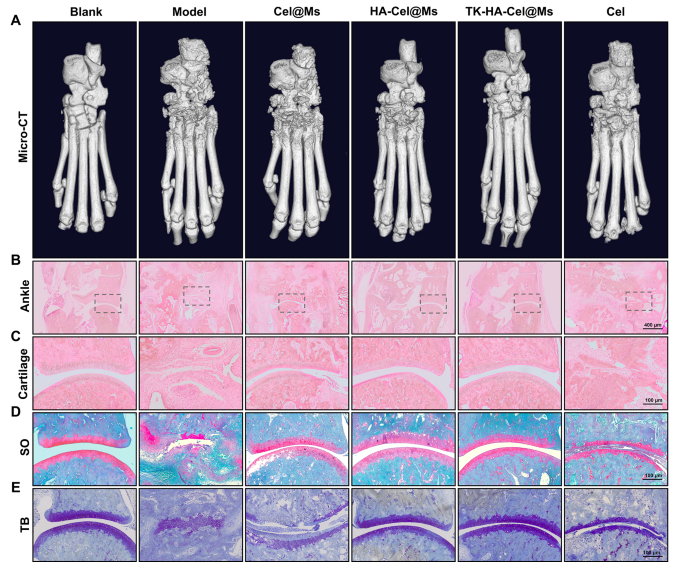
Bone destruction is a major contributor to disability and loss of joint function in RA and is modulated by various factors; it can serve as an important clinical indicator for assessing the severity of arthritis [35, 36]. The knee and ankle joints of rats were scanned using X-ray and micro-computed tomography (micro-CT) to assess the bone damage after treatment in rats with CIA. As shown in Figs. 8A and 9A, and S5, the knee and ankle joints of saline-treated rats with RA showed severe bone damage with rough surfaces and severe bone destruction. Bone erosion was reversed to a certain extent in all treatment groups compared with the model group. This resulted in improved bone quality. The TK-HA-Cel@Ms group demonstrated the most significant effect, with bone quality closest to that of normal rats. The histomorphometric analyses of micro-CT images were also performed to evaluate the extent of bone damage in different groups. Various parameters, such as BMD, BV/TV, BS/TV, Tb.Sp, Tb.Th, and Tb.N, were analyzed. The results showed that TK-HA-Cel@Ms significantly increased BMD, BV/TV, BS/TV, Tb.Th, Tb.N, and decreased Tb.Sp compared with those in rats with CIA (Fig. 8G-L). All these parameters exhibited similar trends, supporting the notion that TK-HA-Cel@Ms slowed the progression of RA and prevented RA-mediated bone destruction and bone erosion.
Micro-CT analysis and histology analysis of the knee joints of rats. (A) Representative micro-CT images of the knee joints of rats; (B–D) HE staining of knee, cartilage and synovium sections of rats in each group; (E) Safranin O staining of cartilage sections of rats in each group; (F) Toluidine blue staining of cartilage sections of rats in each group; (G–L) BMD, BV/TV, BS/TV, Tb.Sp, Tb.Th and Tb.N from joints of rats in different group. Data were presented as mean ± SD (n = 6). #, vs. Blank; *, vs. Model. P < 0.05
Micro-CT analysis and histology analysis of the ankle joints of rats. (A) Representative micro-CT images of the ankle joints of rats; (B–C) HE staining of ankle and cartilage sections of rats in each group; (D) Safranin O staining of cartilage sections of rats in each group; (E) Toluidine blue staining of cartilage sections of rats in each group (n = 6). #, vs. Blank; *, vs. Model. P < 0.05
The expression of macrophage surface markers in synovial tissues of CIA rats was detected by flow cytometry. The results, as shown in Fig. S6, showed that compared with the normal group, the expression of CD86 in synoviocytes of rats in the model group was significantly elevated, and the expression of CD206 was slightly increased, and all the treatment groups decreased the expression of CD86, and increased the expression of CD206 in the CIA rats, in which the treatment effect of TK-HA-Cel@Ms was the most obvious, and the expression of CD86 was down-regulated nearly 3-fold compared with the model group, and the expression of CD206 was increased by 3 times compared with the model group.
The histological analysis was performed to further investigate the protective effect of TK-HA-Cel@Ms on the joints of the CIA rat model. Figs. 8B-D, 9B, C show the results of hematoxylin and eosin (HE) staining of the knee and ankle joints of rats in each group. The cartilage tissue of both knee and ankle joints of normal rats was structurally intact, and the joint surfaces were smooth and neat. In contrast, the joint space of rats with CIA showed cracks and deformations, with a large number of synovial hyperplasia and inflammatory cell infiltration. The knee and ankle joints of rats in each group were stained with Safranin O and toluidine blue, respectively, to evaluate the protective effects of different formulations on inflammation-induced cartilage damage. The cartilages of the ankle and knee joints were clearly stained, with no obvious discoloration of cell staining in the blank control group. In contrast, the cartilage tissue of the knee joint in the model group showed light staining; also, the joint surface was uneven, and the cartilage structure was disorganized (Figs. 8E, F, 9D, E). However, the cartilage staining of rats in each micellar group was significantly darker than that in the model group, and the effect was most obvious in the TK-HA-Cel@Ms group. Moreover, the rats in the TK-HA-Cel@Ms group showed better morphological integrity and less erosive destruction of cartilage surfaces, which was the closest to that in the blank group, suggesting that the TK-HA-Cel@Ms had excellent bone protection and anti-synovial proliferation effects.
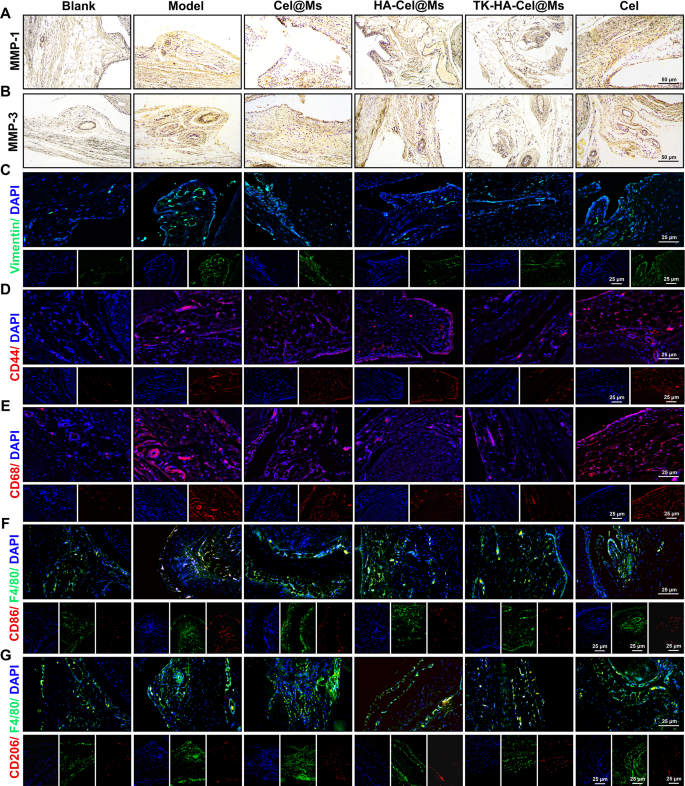
FLSs play a pivotal role in RA pathogenesis, secreting inflammatory cytokines and proteases such as matrix metalloproteinases (MMPs) and causing permanent joint damage [37, 38]. As zinc-dependent endopeptidases, MMPs (e.g., MMP-1 and MMP-3) degrade various proteins in the extracellular matrix (ECM), cause FLSs migration and invasion, and play an important role in the destruction of cartilage and bone [29, 39]. The immunohistochemistry results of MMP-1 (Fig. 10A) and MMP-3 (Fig. 10B) in synovial sections showed a large number of MMP-1 and MMP-3 proteins expressed in the model group compared with the blank control group. In contrast, the expression levels of both proteins decreased in the other treatment groups. The TK-HA-Cel@Ms group had the lowest expression of MMP-1 and MMP-3 proteins, which inhibited FLSs migration and invasion, further reduced cartilage damage and erosion, and protected cartilage. We also chose vimentin, a marker of fibroblasts, to determine the proliferation of FLSs. As shown in Fig. 10C, the immunofluorescence results of vimentin in synovial sections showed that the expression of vimentin was significantly reduced, and FLSs proliferation was inhibited in the TK-HA-Cel@Ms group.
Macrophages are activated during RA pathogenesis, and CD44 and CD68 receptors are highly expressed on their surface [40, 41]. The immunofluorescence staining results of CD44 and CD68 in synovial tissues are shown in Fig. 10D and E. The results showed that CD44 and CD68 were abundantly expressed in the synovium of the model rats. Also, the TK-HA-Cel@Ms significantly inhibited the expression of CD44 and CD86 on the surface of the activated macrophages, effectively alleviating the inflammatory response.
Based on the significant in vitro macrophage phenotypic repolarization ability of TK-HA-Cel@Ms, we further investigated macrophage polarization in inflamed joints using immunofluorescence. As shown in Fig. 10F and G, CD86 fluorescence intensity significantly increased and CD206 fluorescence intensity slightly increased in the synovium of rats in the model group compared with normal rats, suggesting the infiltration of a large number of M1 macrophages into the RA synovium. After treatment with TK-HA-Cel@Ms, the expression of CD86 was significantly reduced, whereas the expression of CD206 significantly increased. This indicated that TK-HA-Cel@Ms promoted the repolarization of M1 macrophages to the M2 phenotype, which was consistent with the results of the in vitro study.
Safety evaluation in vivo
Despite various pharmacological activities of Cel such as anti-inflammatory effects, modulation of macrophage polarization, and inhibition of FLSs proliferation, its potential toxicity remains a concern [42, 43]. Some studies have reported that although effective, Cel can cause damage to multiple organs or tissues and even death [44]. Therefore, HE staining of major organs was performed to observe the morphology of the heart, liver, spleen, lungs, and kidneys to assess the in vivo toxicity of Cel. As observed in Fig. S7, free Cel caused cellular damage and inflammatory cell infiltration in the liver and kidney. The heart, liver, spleen, lung, and kidney tissues of rats in the TK-HA-Cel@Ms group did not show any obvious histological abnormality or lesion, suggesting that TK-HA-Cel@Ms significantly reduced liver and kidney damage, thereby decreasing the hepatorenal toxicity of Cel.