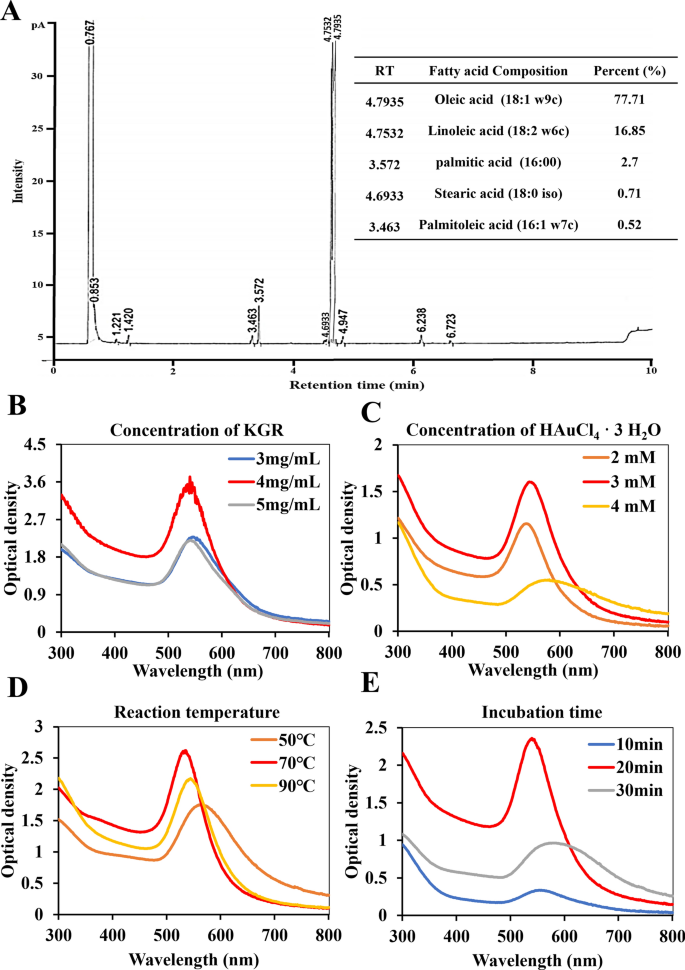
Fatty acid composition of GSO
In our study, we initially analyzed the fatty acid composition of the extracted GSO using GC analysis to identify the specific types of organic compounds present. As illustrated in Fig. 1A, the principal compounds found within GSO include Oleic acid, Linoleic acid, Palmitic acid, Stearic acid, and Palmitoleic acid. Among the organic compounds in GSO, oleic acid constitutes a predominant portion at 77.71%. The second most abundant compound is linoleic acid, constituting 16.85% of the composition. In our results, minor constituents of saturated fatty acids, including Palmitic acid (2.7%) and Stearic acid (0.71%), are present in GSO. Despite the potential impact on cholesterol levels with excessive intake, GSO is generally considered safe for consumption due to the relatively low amounts present. Additionally, GSO contains a very small amount of palmitoleic acid (0.52%). Based on these findings, we found that the extracted GSO was rich in unsaturated fatty acids.
Fatty acid analysis and synthesis of KGR-GNP: A Ginseng seed oil (GSO) fatty acid composition was identified through GC analysis. The GSO contains oleic acid at 77.71%, linoleic acid at 16.85%, palmitic acid at 2.7%, stearic acid at 0.71%, and palmitoleic acid at 0.52%. B–E The optimization process for KGR-GNP synthesis is presented with variations in KGR concentration (3 to 5 mg/mL), Au concentration (2 to 4 mM), temperatures (50 to 90 °C), and incubation time (10 to 30 min)
Optimization, synthesis and physiochemical characterization of KGS-NE
Optimization of KGR-GNP
KGR-GNP was synthesized using the bio-reduction method outlined in the supplementary experimental section. Briefly, KGR was utilized as a reducing agent, and the concentrations of 4 mg/mL for KGR and 3 mM of HAuCl4·3H2O, at 70 °C for 20 min, were optimized to achieve the formation of KGR-GNP. The optimal results for the synthesis of KGR-GNP are presented in Fig. 1B-E and Table S3. As shown in Fig. 2A, the UV–Vis spectroscopy shows the synthesized KGR-GNP absorbance peak at 547 nm, which was confirmed using TEM imaging as shown in Fig. 2B.
Physicochemical characterization of KGS-NE. A The final optimized conditions for synthesizing KGR-GNP were as follows: KGR (4 mg/mL), Au (3 mM), Temperature (70 °C), and incubation time (20 min), with absorbance measured at λ max 547 nm. B Transmission electron microscope (TEM) image of KGR-GNP. C TEM image highlighting KGR-GNP (red arrow) encapsulated by KGS-NE (orange arrow). D Energy-dispersive X-ray (EDX) spectrum confirming the presence of Au in KGS-NE. E Enhanced darkfield microscope (EFM) depicting KGR-GNP inside the KGS-NE through particle reflection. F Selected area diffraction (SAED) displays the crystalline structure of KGS-NE at (111), (200), (220), and (311). G Fourier-transform infrared (FT-IR) spectrum-derived infrared absorption spectrum for confirming the functional groups of KGS-NE
Encapsulation of nanoparticle using GSO
As shown in Figure S1, the optimized GSO at different ratios demonstrated that the 8% ratio had the lowest zeta potential, indicating the highest stability at approximately − 42.54 mV. Consequently, we deliberately chose a 4:2 ratio of Tween 80 and Span 80 to achieve a targeted HLB value of 11.43 for the surfactant blend, which is within the desired range for O/W nanoemulsion [28]. Moreover, the loading efficacy of silydianin into KGS-NE was 94.3 ± 2.6%. The encapsulation content was high, above 90%, as expected in nanoemulsion systems, owing to the hydrophobic nature of silydianin. Some studies have reported that the encapsulation value decreases as the drug concentration increases, suggesting saturation of the system [29,30,31]. The presence of the surface modification monolayer was shown in the TEM image (Fig. 2C), indicating that the KGR-GNP was also successfully encapsulated by the GSO. Nanoemulsions encapsulated were denoted as KGS-NE. Various techniques were employed to confirm the encapsulation of nanoparticles into KGS-NE. Initially, the EDX spectrum, SAED pattern, and EDX were utilized to identify the presence of nanoparticles. We observed the highest Au element peak at 2.1 keV with multiple points and the appearance of a Cu peak at 8 keV was attributed to the copper grid utilized in the EDX analysis (Fig. 2D) [32]. Additionally, the DFM image (Fig. 2E) revealed particle reflections (indicated by red arrows) and the SAED pattern (Fig. 2F) shows crystallographic structures (111, 200, 220, and 311), providing further confirmation of the presence of nanoparticles [33]. FT-IR spectrometry was employed to analyze the presence of functional groups in the KGS-NE (Fig. 2G). Primary peaks were observed at 2922.09 cm−1, which corresponded to alkene C–H stretching. Meanwhile, peaks observed at two different wavelengths, i.e., 1740.24 cm−1, indicated the presence of aldehyde C=O stretching. Additionally, peaks were observed at 1098.07 cm−1, suggesting the presence of C–O stretching in the secondary alcohol.
Stability analysis
Figure 3A presents the Z-average size ranges of KGS-NE including number, volume, and intensity distribution at 202.7 nm, 87.6 nm, and 62.5 nm. The functionalization of KGS-NE showed that the particle size was 153.7 nm, PDI value of 0.25. Moreover, the zeta potential (Fig. 3B) of the KGS-NE was − 44.78 mV, indicating that the KGS-NE is stable. We observed the changes in the synthesized KGS-NE stored at various temperatures over 6 months. As shown in Fig. 3C and D, our findings indicate that the size and stability of KGS-NE remains high at 4 °C for up to 6 months compared to storage at other temperatures. Since KGS-NE is intended for oral administration, it is essential to demonstrate its stability in gastrointestinal fluids. The stability of KGS-NE was evaluated over time in simulated gastric fluid (SGF, composed of 0.2% w/v NaCl in 0.7% v/v HCl, pH 1.2) and simulated intestinal fluid (SIF, consisting of 0.05 M potassium dihydrogen phosphate and 0.02 M sodium hydroxide, pH 7.0). The results indicated that KGS-NE remained stable in both media, with no significant change in particle size observed (Figure S2). These results show that a stable KGS-NE was successfully synthesized as an O/W nanoemulsion with higher stability.
KGS-NE inhibits HCoV-OC43 infection: insights from cell viability, RNA sequencing, and pathway analysis
HCoV-OC43, belonging to the identical viral genus as SARS-CoV and SARS-CoV-2, demonstrates comparable symptoms to those elicited by SARS-CoV-2 [34]. We preliminarily tested the cytotoxicity and viral inhibition effects of KGR-GNP, silydianin, GSO-NE, and KGS-NE against HCoV-OC43. As shown in Figure S3, the non-toxic concentrations of KGR-GNP, silydianin, and GSO-NE were chosen. However, KGR-GNP, silydianin, and GSO-NE did not show better activity than KGS-NE in the non-toxic concentration. Therefore, in further experiments, we focused on studying the antiviral effect of KGS-NE. In a further study, we first examined the antiviral activity of KGS-NE in Vero-E6 cells infected with HCoV-OC43 to explore its efficacy against the virus. In Fig. 4A, a cell viability assay was performed on Vero E6 cells. The MTT assay results revealed that KGS-NE did not show significant cytotoxicity until the concentration reached 10 μg/mL, and the IC50 value of KGS-NE in Vero E6 cells was determined to be 24.92 μg/mL (Fig. 4B). Moreover, as shown in Fig. 4C, different concentrations of KGS-NE were used to quantitatively analyze its effect on HCoV-OC43 N gene production. The results demonstrated that KGS-NE treatments dose-dependently suppressed HCoV-OC43 replication, with viral RNA levels reduced by 98% at 0.5 μg/mL. This result indicated that KGS-NE exhibits antiviral effects in HCoV-OC43-infected Vero E6 cells. Subsequently, we conducted an RNA sequencing analysis of the differentially expressed genes (DEGs) involved in the Vero E6 cells. This analysis aimed to explore the potential of KGS-NE on antiviral and anti-inflammatory effects and its underlying mechanism. As shown in Fig. 4D, treatment with KGS-NE resulted in the DEGs of 145 down-regulated and 178 up-regulated significant genes, as compared to the HCoV-OC43 infection group. The DEGs data was used to show the distribution of genes that were differentially expressed with a fold change of greater than 1.2 or less than 0.8, and a p-value less than 0.05.
Effect of KGS-NE treatment on Vero E6 cells infected by human coronavirus OC43 (HCoV-OC43). A, B Cell viability in Vero E6 cells treated with KGS-NE, including the determination of IC50 value. C RNA levels in HCoV-OC43-infected Vero E6 cells treated with KGS-NE. D Visualization of up-and down-regulated Differential Gene Expressions (DEGs) using a volcano plot. E Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis indicating significant signaling pathways. F Heatmap representation of DEGs involved in the ABC transporters signaling pathway. G Gene Ontology (GO) analysis of biological processes (BP), cellular component (CC), and molecular interactions (MF)
The Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment bubble plots were utilized to summarize the correlation between significant pathways. Figure 4E and Table S4 provided the KEGG mapping of significant genes and their corresponding fold enrichment values. Our study identified several significant signaling pathways, among which ABC transporters exhibited the highest enrichment score, indicating that the ABC transporter’s signaling pathway might serve as the primary regulator. Moreover, as shown in Fig. 4F, the genes associated with the ABC transporters are visualized through a heatmap. This heatmap illustrates the regulation of ABC genes, including their subfamilies, under the influence of KGS-NE treatment and HCoV-OC43 infection. The result shows that the ABC transporters family gene regulations were reversed by KGS-NE treatment. Notably, the ABC transporters subfamily F had significantly opposite regulation among these groups. Therefore, the observed differential regulation of ABCF2 and ABCF3 genes could be indicative of the critical function between the host and the virus. The downregulation after treatment suggests a potential positive response to the intervention by KGS-NE.
Additionally, comprehensive Gene Ontology (GO) enrichment analyses were performed to evaluate the biological functions of all identified DEGs. The GO analysis, encompassing Biological Processes (BP), Cellular Component (CC), and Molecular Function (MF), is presented in Fig. 4G. Within BP, the most significantly enriched genes were associated with catabolic and metabolic pathways. In CC, peroxisomes and microbodies exhibited a similar number of genes with notable enrichment scores. MF, ATPase-coupled transmembrane, Primary active transmembrane, and ATPase activity demonstrated the highest significant enrichment scores. Notably, when comparing BP, CC, and MF the MF shows the highest number of enrichment scores. The GO fold enrichment values of signaling pathways were presented in Tables S5, S6, and S7. Collectively, the Gene Ontology (GO) results provide a comprehensive depiction of the molecular intricacies influenced by KGS-NE treatment including a substantial impact on cellular processes related to energy metabolism and energy-dependent transmembrane transport and ATP hydrolysis.
KGS-NE attenuates lung tissue damage in SARS-CoV-2 spike RBD protein induction in mice
Using SARS-CoV-2 spike RBD protein in mice allows for immune response studies and specific investigations without causing a full COVID-19 infection in mice, as they are not naturally susceptible to SARS-CoV-2. This approach aids in understanding immune responses, developing vaccines, and exploring virus-host cell interactions. After confirming the antiviral efficacy of KGS-NE in Vero E6 cells, additional validation of its effects was evaluated using the SARS-CoV-2 spike RBD protein. To explore the in vivo immune response potential of KGS-NE, mice were orally administered KGS-NE for two days (pre-infection). Subsequently, on the third day, after a 5 h interval from the oral administration of KGS-NE, mice were intratracheally inoculated with SARS-CoV-2 spike RBD. As depicted in Fig. 5A, the treatment regimen was maintained for the next four days (post-infection), totaling six days of the treatment. Following KGS-NE treatment, the severity of acute lung injury was evaluated in the mice at 7 dpi. Histological analysis (Fig. 5B) of lung tissues via H&E staining revealed that RBD protein led to the infiltration of inflammatory cells and thickening of alveolar walls, indicating severe damage to lung tissues. Moreover, the inflammatory score indicates that oral administration of KGS-NE was able to inhibit these conditions and mitigate the adverse effects of RBD protein in a dose-dependent manner. The observed recovery was approximately 56% at the highest dose of 40 mg/kg. The results suggest that administration with KGS-NE alleviates lung injury induced by the RBD protein.
Effect of KGS-NE in SARS-CoV-2 spike receptor binding domain (RBD) protein-induced C57BL/6 mice. A Schematic representation of mice treatment groups. Mice were categorized into five groups: Group 1: Sham group; Group 2: RBD spike protein-induced group; Group 3: RBD spike protein-induced with KGS-NE (10 mg/kg) treatment group; Group 4: RBD spike protein-induced with KGS-NE (20 mg/kg) treatment group; and Group 5: RBD spike protein-induced with KGS-NE (40 mg/kg) treatment group. B Histopathology staining was performed to determine the pathology in the lungs, and the inflammatory score was calculated. C IF staining of ACE2, CD68, and Iba-1 where the intensity is shown in the bar graph. Crosshatch marks indicate significant differences between sham and SARS-CoV-2 RBD, and asterisks indicate significant differences between SARS-CoV-2 RBD and each group. *, # p < 0.05, **, ## p < 0.01, and ***, ### p < 0.001
Angiotensin-converting enzyme (ACE) is a central component of the renin-angiotensin system, crucial for blood pressure and fluid homeostasis. In the context of SARS-CoV-2, the RBD protein binds to ACE2 (Angiotensin-Converting Enzyme 2) as the primary mechanism for cellular entry, contributing to the development of lung injury [35]. Therefore, we investigated whether KGS-NE activates the immune response by affecting the binding of RBD protein to ACE2. Based on the IF staining results shown in Fig. 5C, the RBD protein enhanced the levels of ACE2 expression. In contrast, KGS-NE administration suppressed ACE2 expression by 72%, even at a low concentration of 10 mg/kg. In addition, Ionized calcium-binding adapter molecule 1 (Iba1) and Cluster of Differentiation 68 (CD68) both are commonly used markers in IF to identify the presence of macrophages, which is vital to the immune response of the lungs. Our IF staining results for Iba-1 and CD68 show that the expression was higher in the group treated with RBD protein than in the sham group indicated. After the administration of KGS-NE, it suppressed the Iba-1 and CD68 expression. These results suggest that KGS-NE could prevent acute lung injury caused by the RBD protein through the modulation of ACE2, Iba-1, and CD68. This could indicate a potential mitigation of an overactive or dysregulated immune response, thereby reducing the risk of excessive inflammation or tissue damage.
Moreover, the expression of ACE2, Iba-1, and CD68 was further confirmed through immunoblotting. As depicted in Fig. 6A and B, RBD induction dramatically upregulated the expression of ACE2, Iba-1, and CD68 proteins. Conversely, KGS-NE administration significantly inhibited this upregulation. These collective results strengthen the impact of KGS-NE on immune regulators (ACE2, Iba-1, and CD68), highlighting its potential in modulating immune responses linked to RBD-induced protein expression. In addition to the observed impact on ACE2, Iba-1, and CD68 expression, the pro-inflammatory cytokines (IL-6 and IL-1β) were also evaluated. This analysis could enhance our understanding of KGS-NE immunomodulatory effects in response to RBD induction. Our results show that these proteins were significantly overexpressed by RBD induction, whereas treating with KGS-NE suppressed these expressions. Notably, IL-1β protein expression was suppressed by approximately 89% at the highest dose compared to the infection group. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) serves as a crucial transcriptional activator of cytokines implicated in the innate immune response, such as IL-6 and IL-1β. Beyond its prominent role in innate immunity, NF-κB also plays significant functions in the adaptive immune system. In this study, we assessed the expression of NF-κB protein upon RBD induction. The results indicate a significant upregulation of NF-κB by RBD induction, and KGS-NE exhibited a dose-dependent suppression of its expression. These findings support KGS-NE diverse immunomodulatory impact on key cytokines in the immune response.
Effects of KGS-NE on inflammatory regulators. A Demonstrates that KGS-NE treatment suppressed ACE2, Iba-1, CD68, IL-6, p-NF-κB/NF-κB through protein expression. B A bar graph represents the protein expression analyzed using Image J. Crosshatch marks indicate significant differences between sham and SARS-CoV-2 RBD, while asterisks indicate significant differences between SARS-CoV-2 RBD and each group. *, # p < 0.05, **, ## p < 0.01, and ***, ### p < 0.001
KGS-NE modulates antiviral and anti-inflammatory effects in SARS-CoV-2-infected Syrian hamsters
Syrian hamster was chosen as the experimental animal model to evaluate the feasibility of KGS-NE to inhibit SARS-CoV-2. The Syrian hamsters are small mammals that have been widely used as a model for infection with respiratory viruses, including SARS-CoV-2, influenza viruses, and adenoviruses. In this study, we conducted an assessment to investigate the impact of KGS-NE on SARS-CoV-2-induced pneumonia. The assessment was conducted employing a Syrian hamster model subjected to intranasal infection with SARS-CoV-2, followed by therapeutic intervention utilizing varying dosage levels of KGS-NE. KGS-NE was orally administered (1–6 dpi) after SARS-CoV-2 infection, as shown in Fig. 7A. The weight loss and virus replication number of hamsters were assessed at 4 dpi and 7 dpi, respectively. As depicted in Fig. 7B and C, hamsters infected with SARS-CoV-2 exhibited a decrease in body weight. However, the dose-dependent administration of KGS-NE could alleviate the effects of SARS-CoV-2 infection. Further, the lungs of the hamsters were dissected to examine the effect of KGS-NE treatment against SARS-CoV-2 infection.
Effect of KGS-NE in SARS-CoV-2 infected Syrian hamsters. A Schematic representation of Syrian hamster treatment groups. (B, C Body weight changes in grams and percentage after infecting SARS-CoV-2 and KGS-NE administration. D, E RT-PCR was used to estimate the viral load of RdRp and E gene. Crosshatch marks indicate significant differences between sham and SARS-CoV-2 RBD, while asterisks indicate significant differences between SARS-CoV-2 RBD and each group. *, # p < 0.05, **, ## p < 0.01, and ***, ### p < 0.001
The RNA-dependent RNA polymerase (RdRp) and envelope (E) genes are essential targets for the detection and diagnosis of SARS-CoV-2 infections [36]. Subsequently, we evaluated the RNA copy number of the SARS-CoV-2 specific sequence while targeting the RdRp and E genes to determine whether KGS-NE can prevent the replication of SARS-CoV-2 in lung tissue. As shown in Fig. 7D and E, the expression of RdRp and E genes increased significantly in the SARS-CoV-2 infection, whereas the administration of KGS-NE slightly suppressed the expression of RdRp and E genes.
As shown in Fig. 8A, inflammation was observed in the lungs of SARS-CoV-2-infected hamsters, and these abnormalities were suppressed by administration of KGS-NE. Specifically, lung congestion and swelling escalated over time in SARS-CoV-2-infected hamsters. In contrast, the KGS-NE group demonstrated the ability to reduce this congestion and swelling in a dose- and time-dependent manner. In addition, as shown in Fig. 8B, H&E staining was performed on the lung tissue of hamsters to evaluate the pathological changes in lung tissue caused by SARS-CoV-2 infection. Pathological lung tissue inflammation (red arrow), such as alveolar damage, vascular changes, fibrosis, and morphological disruption, was observed in SARS-CoV-2 infection and was dose-dependently attenuated by KGS-NE administration.
Effect of KGS-NE on lung morphological changes in Syrian hamsters infected with SARS-CoV-2. A Morphology of the dissected lungs in 4 and 7 dpi along with the weight. B Histopathology (H&E) staining performed to assess lung pathology and calculate the inflammatory score. Crosshatch marks indicate significant differences between sham and SARS-CoV-2 infection, and asterisks indicate significant differences between SARS-CoV-2 infection and each group. *, # p < 0.05, **, ## p < 0.01, and ***, ### p < 0.001
To substantiate the inhibitory effects of KGS-NE on the inflammatory response induced by SARS-CoV-2, a qRT-PCR examination was conducted to scrutinize the regulatory impact of KGS-NE on the gene expression of pro-inflammatory cytokines, including NF-κB, IL-1β, IL-6, MCP-1, and TNF-α (Fig. 9). The results unveiled an elevation in the levels of pro-inflammatory cytokines such as NF-κB, IL-1β, IL-6, MCP-1, and TNF-α in hamsters infected with SARS-CoV-2, indicative of an augmented immune response and an inflammatory cascade initiated by viral infection. The intrusion of the coronavirus triggers the activation of the host immune system, prompting cellular synthesis of these pro-inflammatory factors as a defensive measure against infection. In contrast, administration of KGS-NE demonstrated a capacity to down-regulate the gene expression associated with pro-inflammatory cytokines, signifying its potential to attenuate the heightened inflammatory response induced by viral infection. Furthermore, KGS-NE exhibited a substantial reduction in the gene expression of ACE2 in hamster lung tissue. At 4 days post-infection (dpi), there was a 46% reduction, and at 7 dpi, there was a 58% reduction, demonstrating a time-dependent and dose-dependent manner. This observation suggests that KGS-NE may attenuate cellular susceptibility to viruses by diminishing ACE2 gene expression, thereby decelerating the process of virus invasion into host cells. Furthermore, as shown in Figure S4, KEGG pathway analysis identified significant expression of ABC transporter genes, particularly ABCF2 and ABCF3, which were confirmed through qRT-PCR analysis. In summary, these findings collectively endorse the potential of KGS-NE to modulate immune responses and alleviate pulmonary inflammation in hamsters during SARS-CoV-2 infection.
Effect of KGS-NE on lung inflammation in Syrian hamsters infected with SARS-CoV-2. A–F mRNA expression of inflammatory cytokines (IL-6, IL-1β, TNF-α and MCP1), and suppression of ACE2 and NF-κB in KGS-NE administrated group. Crosshatch marks indicate significant differences between sham and SARS-CoV-2 infection, and asterisks indicate significant differences between SARS-CoV-2 infection and each group. *, # p < 0.05, **, ## p < 0.01, and ***, ### p < 0.001
In vivo toxicity assessment of KGS-NE administration
The toxicity assessment of drugs in the serum and liver is critical because these organs participate in drug metabolism and elimination [37]. In this study, the toxicity of KGS-NE was investigated via oral administrations of different concentrations (10–80 mg/kg) to C57BL/6 mice aged eight weeks for 14 days (Fig. 10A). The effect of KGS-NE treatment on the body weight of the mice was evaluated, and the results (shown in Fig. 10B) indicate no significant change in the body weight. Liver histology was assessed via H&E staining (Fig. 10C). Normal liver tissue typically displays cords of hepatocytes with a central vein and sinusoids, and hepatocyte nuclei are typically round or oval with prominent nucleoli. H&E staining showed hepatocyte swelling, necrosis, and inflammation, suggesting liver disease or toxic injury. However, in the present study, KGS-NE (80 mg/kg) did not induce any of these changes, indicating that its non-toxicity to the liver. ALT and AST levels were quantified to evaluate liver function and identify liver injury, whereas ALP and LDH levels were investigated as potential indicators of liver disease. The results are presented in Fig. 10D. The enzyme activities were similar in the non-treatment group, indicating that prolonged KGS-NE administration did not induce significant toxicity. In general, these results suggest that the administration of KGS-NE does not affect liver damage. Moreover, to address concerns about the immune response, we assessed the proportion of immune cells in mice. Specifically, we analyzed the effect of KGS-NE on RAW 264.7 macrophage cells, focusing on cell proliferation. As shown in Figure S5, the results showed no significant changes in cell proliferation after treatment with KGS-NE (40 mg/kg), indicating that KGS-NE does not have toxic effects on immune cells. Overall, these findings demonstrate that KGS-NE is safe with respect to liver function and immune cell health.
In vivo toxicity evaluation of KGS-NE in C57BL/6 mice. A Schematic representation of KGS-NE administration in C57BL/6 mice. B Changes in body weight during experimental period were insignificant; C Histopathological (H&E) examination of liver tissue showed no indications of damage following saPGS-NE administration. D Serum tests for liver function markers, including ALT, ALP, AST, and LDH, showed no evidence of toxicity. Crosshatch marks indicate significant differences between each group. *, # p < 0.05, **, ## p < 0.01, and ***, ### p < 0.001