Design and synthesis
The synthetic route toward AIE motif-functionalized NIR-II dyes is illustrated in Scheme S1 and supporting information. Generally, the accessible nucleophilic substitution of the meso-position chlorine in NIR-C12 with the thiol group of mercaptobenzoic acid yielded carboxylic acid-functionalized NIR-C12. The obtained compound NIR-C12-COOH underwent an amidation coupling reaction between carboxylic acid groups and amino group-functionalized AIE motifs, including triphenylamine (TPA), tetraphenylethylene (TPE), and N1-phenyl-N1-(4-(1,2,2-triphenylvinyl)phenyl)aniline (TPETPA), resulting in the designed NIR-II cyanine dyes C12-TPA, C12-TPE, and C12-TPAE (Fig. 1a). As shown in Figure S22a, as compared to the NIR-C12 molecules with absorbance and emission around 1030 and 1060 nm, the C12-TPA, C12-TPE, and C12-TPAE molecules all presented absorbance to 1049 nm and emission peaks at approximately 1100 nm (Figure S22b), suggesting that the nonconjugated AIE motifs did not interfere with the conjugation strength of the cyanine molecules. To further study the fluorescence properties of dyes in aggregates, these molecules were subsequently dissolved in tetrahydrofuran (THF) and dispersed in water/THF with different water volume ratios. As shown in Figures S22c-f, similar to NIR-C12, the C12-TPA, C12-TPE, and C12-TPAE molecules all exhibited aggregation quenching. Therefore, it is necessary to modify or encapsulate the materials in a certain way to inhibit their quenching effect and apply them to biological experiments.
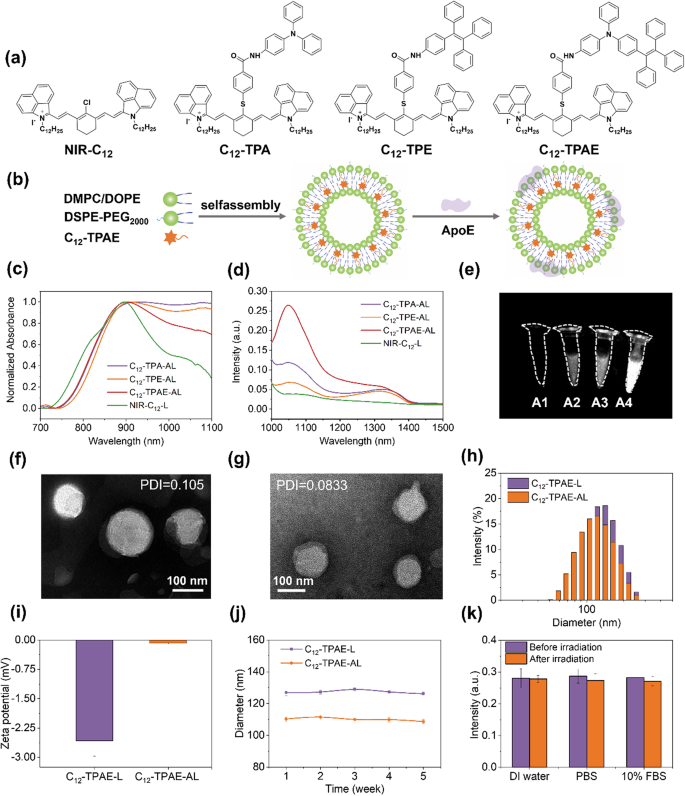
(a) Chemical structures of NIR-C12, C12-TPA, C12-TPE, and C12-TPAE. (b) Schematic illustration of the steps for the preparation of C12-TPAE-AL. (c) Normalized UV‒Vis‒NIR absorption of the samples. (d) Fluorescence emission of NIR-C12-L, C12-TPA-L, C12-TPE-L, and C12-TPAE-L (concentration = 150 µg/mL) in PBS (λex = 808 nm). (e) NIR-II fluorescence images (1000LP, 1000 ms) of samples (concentration = 150 µg/mL) under 808 nm laser irradiation at 60 mW. Transmission electron microscopy images of C12-TPAE-L (f) and C12-TPAE-AL (g). (h) Dynamic light scattering analysis and (i) zeta potential of C12-TPAE-L and C12-TPAE-AL. (j) Size stability of C12-TPAE-L and C12-TPAE-AL over 5 weeks. (k) Fluorescence stability of C12-TPAE-AL in DI water, PBS, and 10% FBS before irradiation and after irradiation
The large apolipoprotein E (ca.34 kDa), especially high-density lipoproteins (HDLs), exist endogenously in the morphology of nanostructured particles with small sizes (< 25 nm). They consist of different proteins, such as apolipoprotein A1(ApoA1), and diverse lipids, such as phospholipids and cholesterol esters, which endow them with good colloidal stability, active targeting capability, and appealing bilayer structures with the potential to encapsulate and deliver imaging or therapeutic agents to their desired destinations and achieve deep tissue penetration [38,39,40,41,42,43]. Importantly, lipoproteins, such as low-density lipoprotein receptor (LDLR) and LDLR-related proteins (e.g., LRP-1 and LRP-2), have demonstrated good selectivity and efficient targeting capabilities for glioma via the overexpression of receptors on glioma [38,39,40,41,42]. However, their large size leads to significant challenges in large-scale production, conjugation, purification, stability, and even potential immunogenic concerns [38,39,40,41,42]. Moreover, the short ApoE peptide is less immunogenic, easy to synthesize, and amenable to different chemical manipulations, and the nanoplatforms decorated with the ApoE peptide display properties similar to those of endogenous natural lipoproteins and could contribute to good BBB permeability and glioma targetability [38,39,40,41,42]. In this work, we formulated dye (C12-TPA、C12-TPE、C12-TPAE)-loaded PEGylated liposomes with 1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine (DMPC), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and 1,2 distearoyl-snglycero-3-phosphoethanolamine-N-Methoxy polyethyleneglycol-2000 (DSPE-PEG2000) via a thin film dispersion method, yielding C12-TPA-L, C12-TPE-L and C12-TPAE-L NPs. After adsorption treatment with small apolipoprotein E peptide (sequence: LRKLRKRLLLRKLRKRLLC, ApoE, ca. 2.5 kDa), which has affinity for phospholipids, lipoprotein-mimicking-NPs (C12-TPA-AL, C12-TPE-AL and C12-TPAE-AL) were collected, respectively. It was found that around 92% of ApoE peptide added to the mixture was inserted into the lipid bilayer of lipoprotein-mimicking NPs, including C12-TPA-AL, C12-TPE-AL, and C12-TPAE-AL (Figure S23a, supporting information). In order to explore whether the addition of ApoE affects the photophysical properties of liposomes, as shown in Figures S23b-c, the near-infrared absorption and fluorescence emission of C12-TPAE-AL before and after the introduction of ApoE remained essentially unchanged, and good photophysical properties were maintained.
As shown in Fig. 1c-d, C12-TPA-AL, C12-TPE-AL, and C12-TPAE-AL exhibited similar profiles in the UV/vis and fluorescence spectra, with absorption maxima and emission peaks at approximately 900 and 1050 nm, respectively. Importantly, the fluorescence intensity of C12-TPAE-AL was approximately 2.45-, 1.72- and 3.94-fold that of C12-TPA-AL, C12-TPE-AL, and NIR-C12-L, respectively (Fig. 1c-d). This is mainly attributed to the three AIE motifs with different steric hindrance effects (TPA < TPE < TPATPE), which suppressed π-π stacking-induced fluorescence quenching. The quantitative fluorescence quantum yields (QYs) of C12-TPA-AL, C12-TPE-AL, and C12-TPAE-AL were calculated to be 0.81%, 0.80%, and 1.56%, respectively, with NIR-C12-L (QY = 0.5774%) as the reference [44] (Figure S24, supporting information). Since the generally high brightness of the imaging agents contributes to better imaging fidelity, the sample C12-TPAE-AL with the highest QY was selected for further in vitro and in vivo studies.
As shown in Fig. 1f-i, the C12-TPAE-L liposome sample presented a spherical morphology with sizes of 129.5 and 114.9 nm by transmission electron microscopy (TEM) and dynamic light scattering (DLS), respectively, and a surficial negative charge of -2.58 mV. After decoration with the ApoE peptide, C12-TPAE-AL was determined to be 110.4 and 108.59 nm in size by TEM and DLS, respectively, with a surficial negative charge of -0.08 mV. These results suggest that ApoE3 as a negative-charged protein, was successfully inserted into the surface of liposome and it did not have a significant impact on the morphology of liposomes [45]. The polydispersity indices (PDIs) determined via DLS revealed that the polydispersity indices (PDIs) of C12-TPAE-L and C12-TPAE-AL were 0.104 and 0.083, respectively, indicating the uniform size of the NPs [46]. Furthermore, the stability tests revealed that both C12-TPAE-L and C12-TPAE-AL remained stable for 5 weeks, indicating good colloidal stability (Fig. 1j). In addition, C12-TPAE-AL was placed in different media (deionized water, PBS, and 10% FBS) and irradiated with a laser of 1064 nm (1.0 W/cm2) for ten minutes. There were no obvious fluorescence intensity changes, suggesting good fluorescence stability (Fig. 1k).
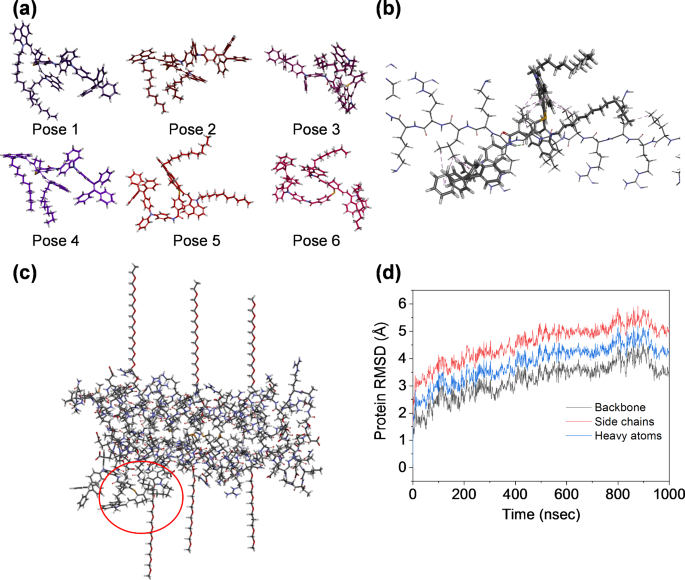
To improve the understanding of the working mechanism for the enhanced brightness of C12-TPAE dyes after encapsulation in liposomal bilayers, the interactions between C12-TPAE and the liposomes were investigated by molecular docking and molecular dynamics modelling [47,48,49,50]. As shown in Fig. 2, the docking score was constructed to qualitatively and quantitatively study the binding sites and poses of C12-TPAE in the hydrophobic cavities of the liposomal bilayer, which inhibits the rotation of the AIE motifs with enhanced brightness. The low and negative binding energies indicate a spontaneous and energetically favorable process between the molecule (C12-TPAE) and receptor (liposome), which suggests that there exists tight binding between C12-TPAE molecules and liposomal components. Notably, the high docking score of pose 1 (Fig. 2a) is the dominant pattern for C12-TPAE molecules with a docking assembly as shown in Fig. 2b. During molecular simulation, C12-TPAE acts as ligands to dock into liposomes, in which the root mean square deviation (RMSD) value gradually stabilized and fluctuated around a certain value in the system (Fig. 2c-d). This means that the dye-liposome system gradually reached equilibrium within a certain period. The modelling results suggest that liposomes processed with thin-film extrusion method offer a hydrophobic environment for brightness enhancement and water-dispersion for C12-TPAE dyes in the aqueous environment.
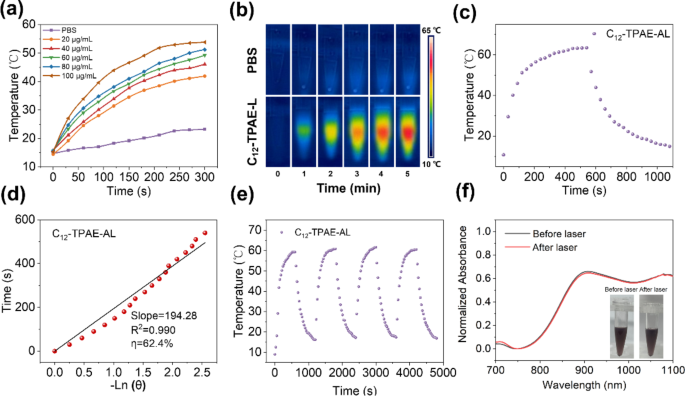
To study the photothermal effects, an NIR thermometer was applied to continuously record the temperature changes of sample C12-TPAE-AL at different concentrations (20, 40, 60, 80, and 100 µg/mL) under 1064 nm laser irradiation (1.0 W/cm2), while PBS was used as a control (Fig. 3). It was noticed the PBS temperature changed only slightly from 15 °C to 22 °C after 5 min of continuous laser irradiation, whereas the C12-TPAE-AL (20 µg/mL) temperature increased from 15 °C to above 42 °C under the same conditions, indicating that C12-TPAE-AL has good photothermal heating performance. For another example, the hyperthermia temperature of C12-TPAE-AL (120 µg/mL) rapidly increased and reached a plateau of 63.4 °C within 10 min of continuous laser irradiation, and the photothermal conversion efficiency was calculated to be 62.4% (Fig. 3c-d), which is much higher than that of many organic and inorganic photothermal agents [51]. In addition, both the cyclic photothermal heating and cooling processes and the UV/vis spectra of C12-TPAE-AL before and after continuous 1064 nm laser irradiation were studied to examine the photostability of the NPs. The results showed that the NPs could be reversibly photothermally heated for four cycles without obvious changes (Fig. 3e), and the absorption spectra remained stable even after 10 min of laser irradiation, indicating good photostability. Taken together, these results indicate that C12-TPAE-AL could quickly, efficiently, and stably convert NIR light energy to thermal energy through a nonradiative decay pathway, which would benefit the in vitro and in vivo long-term and repeated treatment of cancers.
(a) Temperature changes in PBS and C12-TPAE-AL with different concentrations over different times under 1,064 nm laser irradiation (1 Wcm− 2). (b) Thermal images of PBS and C12-TPAE-AL (100 µg/mL) within 5 min under 1064 nm laser irradiation. (c) Photothermal performance of C12-TPAE-AL (120 µg/mL) after cooling to room temperature. (d) The corresponding linear analysis of C12-TPAE-AL. (e) Photothermal stability study of C12-TPAE and C12-TPAE-AL (120 µg/mL) during four cycles of heating‒cooling processes. (f) Comparison of UV-Vis-NIR absorption of C12-TPAE-AL before and after 10 min of 1064 nm laser irradiation (1 Wcm− 2) inset: picture of C12-TPAE-AL before and after laser irradiation)
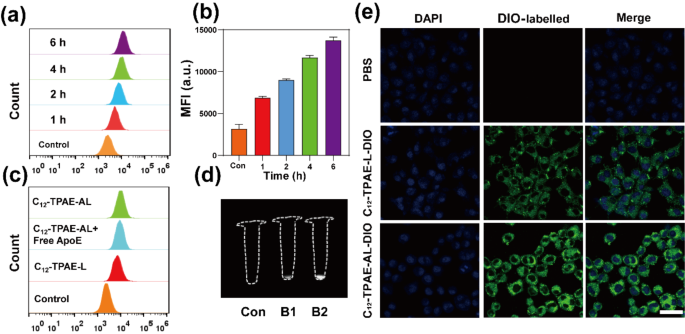
Before in vivo examination, we studied the in vitro cellular uptake, cytotoxicity, and photothermal efficacy of the lipoprotein-mimicking NPs. To investigate and visualize the active tumor-targeting effects of ApoE decoration, we coloaded C12-TPAE with DIO (a commercial cell membrane marker) in the NPs, yielding C12-TPAE-L-DIO and C12-TPAE-AL-DIO NPs, respectively, and studied their cellular update in U87 cells (Fig. 4). First, flow cytometry was used to quantitatively study the cellular uptake ability of C12-TPAE-AL. As shown in Fig. 4a, the uptake of C12-TPAE-AL increased with increasing incubation time, and the results after 4 h and 6 h of incubation were similar. Thus, 6 h of incubation was estimated to be the time point of complete uptake. Subsequent cell experiments used this time point as the drug uptake time in U87 cells. Similarly, the corresponding average fluorescence intensity confirmed this finding (Fig. 4b). As shown in Fig. 4c, DIO-labelled C12-TPAE-L and DIO-labelled C12-TPAE-AL were then coincubated with U87 cells for 4 h. The results showed that the C12-TPAE-AL group had stronger fluorescence than the C12-TPAE-L group. Moreover, pretreatment of U87 cells with free ApoE for 0.5 h reduced the uptake of the C12-TPAE-AL group to a certain extent. The literature reports that during tumor proliferation, the low-density lipoprotein family receptor (LDLR) on the surface of U87 cells is upregulated, promoting cellular cholesterol uptake through receptor-mediated lipoprotein endocytosis [38]. Therefore, by modifying the surface of liposomes with ApoE, LDLR receptor-mediated lipoprotein uptake is more conducive to the high uptake of C12-TPAE-AL in U87 cells. Importantly, the NIR-II fluorescence imaging instrument revealed that the uptake of C12-TPAE-AL was two times greater than that of C12-TPAE-L (Fig. 4d). Similarly, after 6 h of incubation, the intracellular fluorescence distribution was observed via confocal laser scanning microscopy (CLSM), and the results were consistent with the results of flow cytometry and NIR-II fluorescence imaging. Overall, ApoE-functionalized modified liposomes are more conducive to the internalization of NPs by U87 cells through receptor-mediated endocytosis and are localized in the cytoplasm.
(a) Flow cytometry analysis of U87 cells treated with DIO-labelled C12-TPAE-AL for different incubation times. (b) Flow cytometry quantitative analysis for different incubation times for DIO-labelled C12-TPAE-AL. (c) Flow cytometry quantitative analysis of U87 cells treated with DIO-labelled C12-TPAE-L and DIO-labelled C12-TPAE-AL for 4 h. (d) NIR-II fluorescence images (1000LP, 1000 ms) of U87 cells treated with PBS (Con), C12-TPAE-L (B1), or C12-TPAE-AL (B2) for 6 h under 808 nm illumination (60 mWcm− 2). (e) CLSM images of U87 cells incubated with DIO-labelled C12-TPAE-L or DIO-labelled C12-TPAE-AL for 4 h. Scale bar = 25 μm
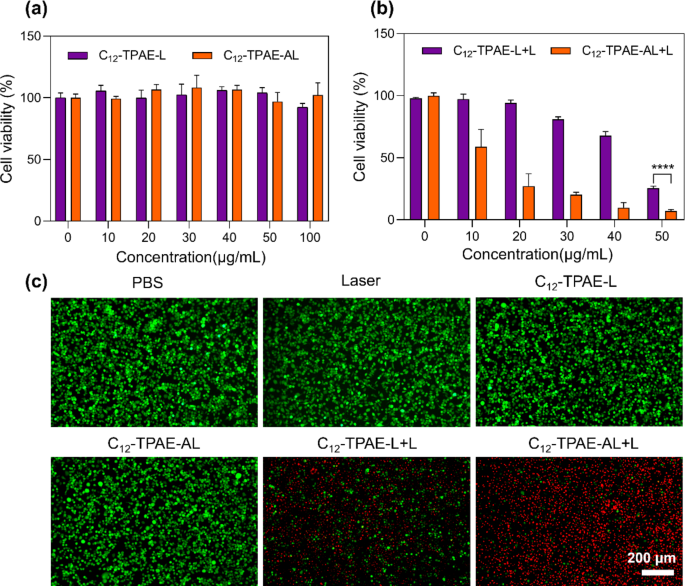
As shown in Figure S25a, after incubation with the liposome blank carrier for 6 h, no significant cytotoxicity was detected in U87 cells, and the cell viability remained above 95%, indicating that the liposome blank carrier has low toxicity and can be used for tumor treatment in vivo. Furthermore, incubation of U87 cells with C12-TPAE-L or C12-TPAE-AL at concentrations as high as 100 µg/mL for 6 h had no significant effect on the viability of U87 cells, suggesting good biocompatibility of the NPs. Notably, 1064 nm laser irradiation (1 Wcm-2) alone did not cause side effects on cell viability. In contrast, the hyperthermia caused by the photothermal effects of the NPs under continuous 1064 nm laser irradiation contributed to severe inhibition of cell growth. Importantly, ApoE decoration on lipoprotein-mimicking NPs contributed to not only increased cellular uptake but also a greater percentage of cell inhibition in the “C12-TPAE-AL + Laser” group than in the “C12-TPAE-L + laser” group. For example, the survival rate of U87 cells treated with 50 µg/mL C12-TPAE-AL was only 7.22%, which was much lower than that of the C12-TPAE-L group (25.52%). Furthermore, the IC50 value for the “C12-TPAE-AL + L” group was approximately 0.039 µg/mL, which is much lower than that of the “C12-TPAE-L” group, with an IC50 of 464 µg/mL, suggesting that the addition of lipoprotein-mimicking NPs with short ApoE decorations is of significant value for enhancing the efficacy of photothermal treatment. To visually evaluate PTT in vitro, calcein (AM, green fluorescence) and propidium iodide (PI, red fluorescence) were used to further differentiate between live and dead cells, respectively (Fig. 5c). Notably, in all of the control groups, there was strong green fluorescence, which further verified the good biocompatibility of the NPs and the minimal side effects caused by 1064 nm laser irradiation alone. Furthermore, there was obviously strong red fluorescence in the “C12-TPAE-AL + L” group, whereas both green and red fluorescence appeared in the “C12-TPAE-L + L” group. This finding demonstrated that ApoE decoration on lipoprotein-mimicking NPs contributed to increased local C12-TPAE concentrations inside cancer cells and enhanced photothermal cytotoxicity (Fig. 5), which was consistent with the above cytotoxicity experiments (Fig. 5a-b and S25). All the results demonstrated that C12-TPAE-AL NPs have high photothermal ablation ability under continuous 1064 nm laser irradiation.
(a) Cytotoxicity of C12-TPAE-L and C12-TPAE-AL to U87 cells at different concentrations for 6 h. (b) Relative viability of U87 cells incubated with different concentrations of C12-TPAE-L and C12-TPAE-AL with 1064 nm laser irradiation at a power density of 1.0 Wcm-2 for 5 min. (c) Fluorescence images of Calcein-AM/PI costained cells subjected to different treatments (PBS, Laser,50 µg/mL C12-TPAE-L, 50 µg/mL C12-TPAE-AL, 50 µg/mL C12-TPAE-L + L, 50 µg/mL C12-TPAE-AL + L) (laser: 1064 nm, 1 Wcm-2, 5 min). The scale bar is 200 μm
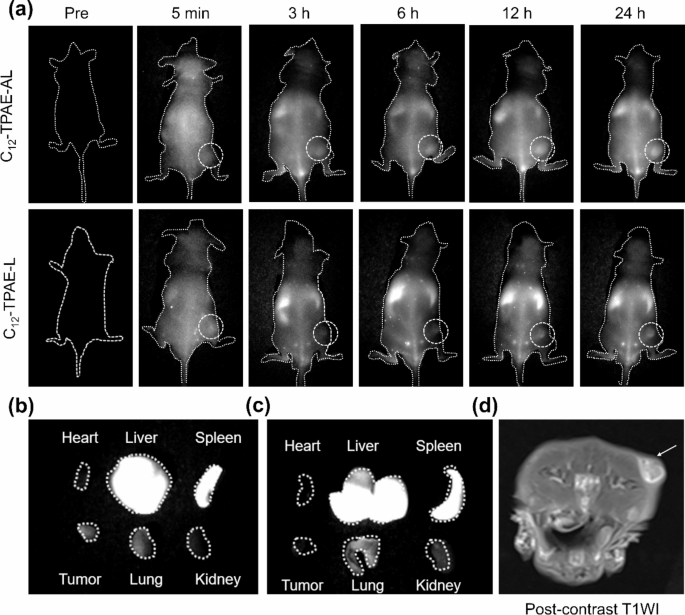
Motivated by the bright fluorescence emission of C12-TPAE-AL in the NIR-II region and excellent phototoxicity toward cancer cells, we further evaluated the effectiveness of these materials for tumor diagnosis and therapeutic applications in vivo. To evaluate tumor growth, tumor-bearing mice were subjected to nuclear magnetic resonance imaging. As shown in Fig. 6d, after injecting a commercial Gd contrast agent (2 mg/kg, 200 µL) through the tail vein, nuclear magnetic resonance imaging of the tumor site was significantly enhanced. The above results show that the subcutaneous tumor model in the mice was successfully established.
To further explore the application prospects of C12-TPAE-AL in in vivo tumor diagnosis and surgical resection, we conducted an NIR-II fluorescence imaging experiment on subcutaneous glioblastoma in vivo to further verify the ability of C12-TPAE-AL to target tumors. C12-TPAE-L and C12-TPAE-AL (10 mg/kg, 200 µL) were injected into tumor-bearing mice through tail vein, and the fluorescence signals were collected at multiple time points after injection. As shown in Fig. 6a, the fluorescence signal at the tumor site in the C12-TPAE-AL group continuously increased with time, approaching a maximum at 12 h after injection, and showed long-term tumor retention. At 24 h after injection, the fluorescence signal at the tumor site gradually decreased. Moreover, compared with that of the C12-TPAE-L group, the fluorescence signal of the C12-TPAE-AL group was greater than that of the C12-TPAE-L group at each time point (Fig. 6 and S26), indicating that ApoE-functionalized liposomes can effectively target the tumor site and, importantly, that effective retention at the tumor site can increase the therapeutic efficacy of PTT. In addition, after 24 h, the mice were euthanized, and the main organs and tumors were collected and subjected to NIR-II fluorescence imaging, which show NP accumulated mainly in liver, spleen, lung and tumor. Taken together, these results suggest that ApoE-modified C12-TPAE-AL has good tumor-targeting capacity, which would benefit photothermal treatment outcomes.
(a) NIR-II fluorescence imaging (1000LP, 1000 ms) of tumor-bearing nude mice (10 mg/kg) at different time points (pre-injection, 5 min, 3 h, 6 h, 12 h, and 24 h) under 808 nm illumination (60 mWcm-2). after tail vein injection of 200 µL of C12-TPAE-L and C12-TPAE-AL. (b) NIR-II fluorescence images of ex vivo organs and tumors of C12-TPAE-AL and (c) C12-TPAE-L. (d) Post-contrast T1-weighted image demonstrates the subcutaneous tumor of mice with MRI (the arrow represents the tumor)
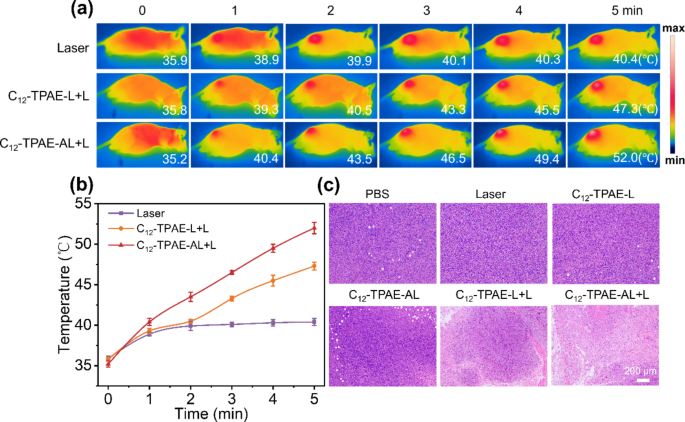
The photothermal effects of C12-TPAE-AL in vivo were monitored by a thermal imaging camera. According to the results of the NIR-II fluorescence imaging (Fig. 6), the time point with the strongest fluorescence signal (12 h after administration) in the subcutaneous tumors was selected for photothermal treatment with a 1064 nm laser. For the subcutaneous glioblastoma model, C12-TPAE-L and C12-TPAE-AL (200 µL, 10 mg/kg) were injected into mice through the tail vein. After 12 h of administration, the tumor sites of the mice were exposed to continuous 1064 nm (1 Wcm-2) laser irradiation for 5 min. As shown in Fig. 7a-b, the temperature of the tumor site for the mice injected with PBS increased only slightly. However, under 1064 nm laser irradiation for 5 min, the temperature of the tumor site in the “C12-TPAE-L” and “C12-TPAE-AL” groups sharply increased to 47.3 and 52.0 °C, respectively, indicating that the better tumor-targeting capacity of C12-TPAE-AL contributed to the higher hyperthermia temperature under laser irradiation than that of C12-TPAE-L. Furthermore, at 4 h after photothermal treatment, some mice were euthanized, and the tumor sites were removed for hematoxylin and eosin (H&E) staining analysis. As shown in Fig. 7c, the sections of the tumor tissues from the PBS, laser, C12-TPAE-L, and C12-TPAE-AL groups presented a morphology of tumor cells without obvious damage. However, the tumor tissues of the C12-TPAE-L + L group were damaged via cell necrosis to a certain extent under laser irradiation. Importantly, the tumor site of the C12-TPAE-AL + L group exhibited much greater damage than the other groups did, suggesting that almost all the tumor cells were killed. These results showed that, compared with the use of NPs or laser irradiation alone, C12-TPAE-AL exhibited outstanding tumor ablation effects under laser irradiation, indicating that it has excellent targeting and photothermal ablation capabilities.
(a) Infrared thermal imaging images of different groups (PBS, C12-TPAE-L + L, and C12-TPAE-AL + L) under 1064 nm (1 Wcm-2) laser irradiation for 5 min. (b) Temperature variation changes of different groups of materials over time at the corresponding times. (c) images of tumors from mice in different groups of materials treated with PTT for 4 h. Scale bar: 200 μm
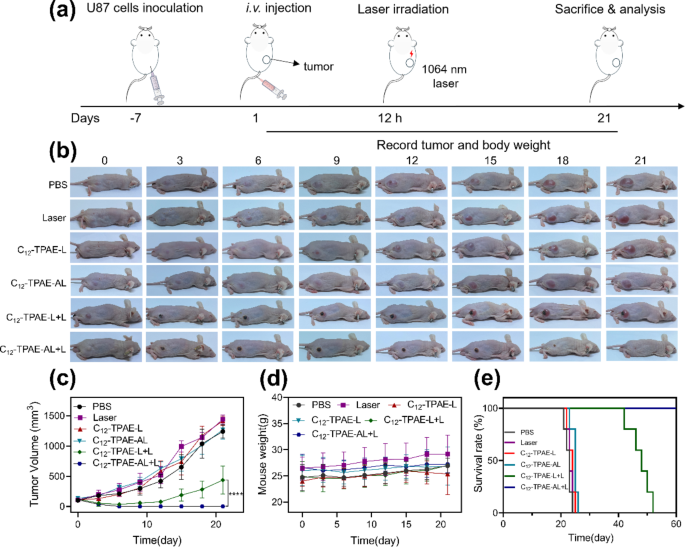
The quantitative analysis of photothermal therapeutic efficacy was performed by monitoring the tumor growth rate and survival rate every 3 days after treatment (Fig. 8), in which the 6 groups of U87 tumor-bearing mice were the “PBS”, “Laser”, “C12-TPAE-L”, “C12-TPAE-AL”, “C12-TPAE-L + L”, and “C12-TPAE-AL + L” groups. The size of the tumors of the mice in all the control groups gradually increased over time and expanded approximately 127-fold on day 21, indicating that neither NP injection alone nor laser irradiation alone can achieve any antitumor efficacy. However, the growth of the tumors in the C12-TPAE-L + L group was inhibited at the beginning of the treatment but relapsed on day 6, with a 50% survival rate up to day 35. Notably, the growth of tumors in mice in the C12-TPAE-AL + L group was completely inhibited, and the tumors did not recur, with a 100% survival rate on day 60. In addition, during the treatment, the weights of the mice in each group did not obviously decrease. These findings suggested that all the treatments in this study did not cause any significant side effects. The above results show that C12-TPAE-AL has good biocompatibility, effective targeting, and high 1064 nm photothermal ablation properties for subcutaneous glioma, which is consistent with the in vitro results.
(a) Schematic representation of the photothermal therapeutic process. (b) photothermal therapy photos of in subcutaneous tumor model, which included PBS, laser, C12-TPAE-L, C12-TPAE-AL, C12-TPAE-L + L and C12-TPAE-AL + L (injection dose = 2 mg/kg, 1064 nm laser, 1 W/cm2). (c) Tumor volume curves, (d) body weights, and (e) survival rates of treated mice subjected to various treatments (n = 5). The data are presented as the mean ± SD. The p values are calculated using two-tailed unpaired t test, *p < 0.05, **p < 0.01, and ***p < 0.001
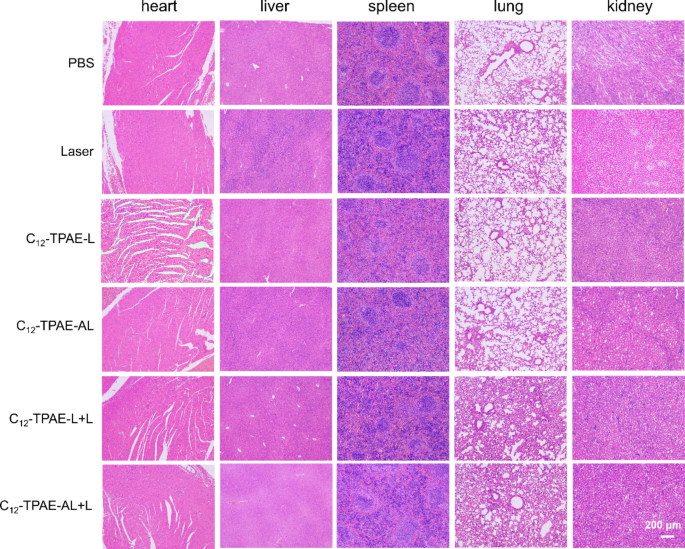
To further investigate the therapeutic efficacy and biocompatibility of these treatments, histological staining was systematically performed. After 21 days of treatment, H&E staining performed on major organs of the mice, including the heart, liver, spleen, lungs, and kidneys (Fig. 9). The H&E staining results of all the groups revealed no obvious changes in the cellular structure. Additionally, there were no significant differences observed in biochemical indicators related to heart, liver and kidney function (Figures S27a-c), including serum lactate dehydrogenase (LDH), alanine aminotransferase (ALT), and blood urea nitrogen (BUN). Taken together, these results show that the cyanine dye-loaded lipoprotein-mimicking NPs have excellent biocompatibility and biosafety.