Characterization
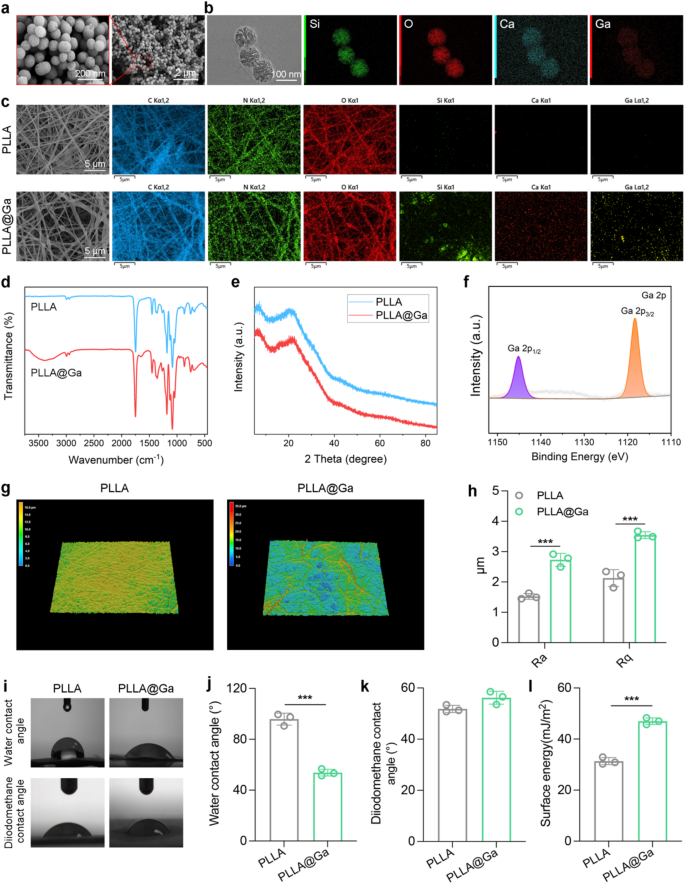
SEM and TEM were utilized to characterize the morphology and elemental composition of the synthesized Ga-MBG. As depicted in Fig. 1a, Ga-MBG appears as uniform spherical particles with an average diameter of approximately 100 nanometers, consistent with TEM observations. TEM images (Fig. 1b) reveal the uniform spherical morphology of Ga-MBG particles with an average diameter of approximately 100 nm. In addition, TEM image clearly shows the mesoporous structure of Ga-MBG. Energy-dispersive X-ray spectroscopy (EDS) mapping confirms the homogeneous distribution of Ga within the MBG framework, indicating successful doping of Ga ions. As demonstrated in Fig. 1c, the successful preparation of Ga-MBG loaded PLLA electrospun fibers resulted in the uniform distribution of Ga-MBG within the fibers. The FTIR spectrum of electrospun membranes is illustrated in Fig. 1d. The original PLLA FTIR absorption spectrum displays C═O and C-O-C stretching vibrations at 1756 and 1182 cm− 1, respectively. Furthermore, the absorption band at 1045 cm− 1 is linked to C-CH3 stretching vibrations, while those at 1087 and 1130 cm− 1 correspond to the symmetric stretching of C-O-C and CH3 rocking modes, respectively [32,33,34]. In contrast, all composite spun membranes show characteristic absorption bands of PLLA. Notably, PLLA@Ga does not exhibit any distinct additional absorption bands compared to PLLA. This may be due to overlapping absorption bands between PLLA and PLLA@Ga, or possibly due to the very low content of nanofillers in the composite material. FTIR results suggest that the chemical structure of PLLA remains unchanged from its original state upon the addition of nanofillers. XRD analysis was conducted to investigate the structural characteristics of PLLA@Ga for further research purposes (Fig. 1e). The peaks observed at 19.7° (broad) and 16.6° were attributed to the 203 and 200/100 planes of PLLA, respectively [35]. The XRD pattern of the composite material revealed the absence of peaks corresponding to the nano filler (Ga-MBGs), suggesting that the nano filler is evenly dispersed within the PLLA matrix or present at a lower concentration compared to PLLA in the composite material. The XPS spectra of Ga 2p indicate that Ga-MBG are embedded within the internal framework of PLLA@Ga (Fig. 1f).
The three-dimensional microscopic images obtained using laser confocal microscopy were utilized to measure surface roughness (Fig. 1g-h). PLLA@Ga exhibited surfaces rougher than PLLA, consistent with the findings from the scanning electron microscopy images. WCA is utilized to determine the surface hydrophilicity of electrospun membranes. Studies have shown that various factors, including cell adhesion and protein absorption, are influenced by the hydrophilicity of biomaterials [36]. Therefore, biomaterials with appropriate hydrophilicity are crucial in a biological environment. In our research, the original PLLANF scaffold had a WCA of 95.8°, indicating that PLLA is hydrophobic (Fig. 1i-j). However, the WCA of the composite membrane incorporating Ga-MBG fibers decreased to 53.8°. Thus, the incorporation of Ga-MBG enhanced the hydrophilicity of the composite membrane, providing PLLA@Ga with improved biocompatibility in terms of cell proliferation and differentiation.
To quantify the surface energy of different samples, the contact angles of diiodomethane were measured (Fig. 1k), yielding values of 51.8°(PLLA) and 56.2° (PLLA@Ga). Furthermore, the surface energy of PLLA and PLLA@Ga samples were measured to be 31.35 and 46.98 mJ/m2, respectively (Fig. 1l). The surface energy of PLLA@Ga is significantly higher than that of pure PLLA, a change primarily attributed to the introduction of Ga-MBG, which enhances the hydrophilicity of the material. This increase in surface energy indicates that the PLLA@Ga composite exhibits greater polarity and hydrophilicity in an aqueous environment, facilitating improved interactions with biomolecules, particularly the adsorption of proteins and the formation of the extracellular matrix (ECM). Research has shown that higher surface energy can provide a more favorable microenvironment for cells, thereby promoting cell adhesion, growth, and proliferation [37]. By facilitating the construction of the extracellular matrix, it can further enhance tissue functional recovery and repair. Moreover, high surface energy materials can also improve signal transduction between cells and the material, which is crucial for the tissue regeneration process. In the context of diabetic wound healing, effective signal transduction at the cell-material interface can promote cell activation and differentiation, thereby accelerating the wound healing process. By modulating the energy state of the material’s surface, the PLLA@Ga composite can effectively improve the wound microenvironment, leading to enhanced wound repair outcomes.
Therefore, based on its excellent surface energy characteristics and biocompatibility, the PLLA@Ga composite emerges as a highly suitable novel dressing for diabetic wound repair. This material can provide superior support for cell growth, offering an ideal therapeutic solution for wound healing in diabetic patients.
Preparation and characterization of Ga-MBG and PLLA@Ga. (a) SEM images of Ga-MBG. (b) TEM and EDS images of Ga-MBG. (c) SEM and EDS mapping images of PLLA and PLLA@Ga. (d) FTIR of PLLA and PLLA@Ga. (e) XRD of PLLA and PLLA@Ga. (f) XPS of PLLA@Ga. (g) 3D-CLSM of PLLA and PLLA@Ga. (h) Surface roughness of PLLA and PLLA@Ga. (i) Water contact angle and diiodomethane contact angles of PLLA and PLLA@Ga. (j) Statistics on water contact angle of PLLA and PLLA@Ga. (k) Statistics on diiodomethane contact angles of PLLA and PLLA@Ga. (l) Surface energy of PLLA and PLLA@Ga
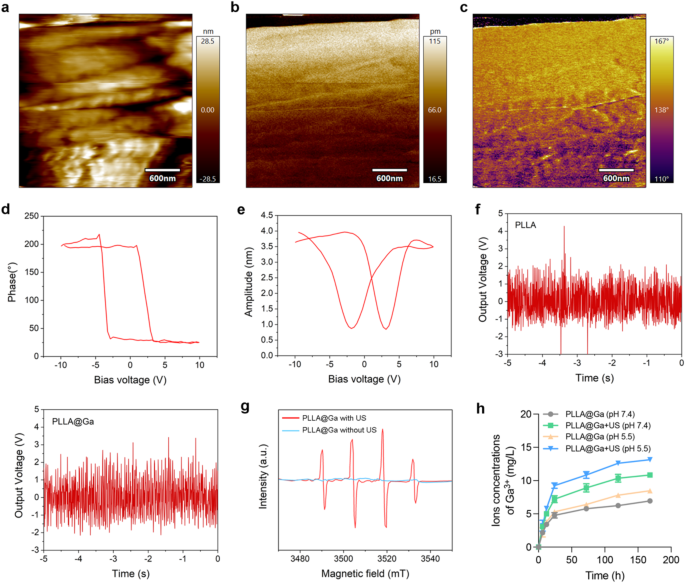
In the PFM mode of atomic force microscopy (AFM), a tip bias of 10 V was applied to conduct localized point-to-point piezoelectric response tests on the sample material, aimed at investigating the piezoelectric characteristics of the material’s surface. Figure 2a illustrates the surface of the selected piezoelectric material. The experimental results presented in Fig. 2b-c indicate that the magnitude of the piezoelectric response on the material’s surface, following the application of the tip bias, was 115 pm and a phase angle of 167°, respectively. This suggests that the material’s surface exhibits a high piezoelectric response capability, further validating the exceptional piezoelectric properties of the PLLA@Ga composite material at the nanoscale. The characteristic hysteresis loop and pronounced butterfly curve observed in the PLLA@Ga samples in Fig. 2d-e reveal the material’s spontaneous polarization ability under the influence of switching voltage, indicating favorable electrical behavior. Additionally, the material continues to demonstrate a robust piezoelectric effect under ultrasonic mechanical forces, showcasing inherent spontaneous polarization characteristics, thereby defining the material’s non-zero remnant polarization or ferroelectric properties. These piezoelectric and polarization characteristics provide significant evidence for the material’s potential applications in the biomedical field, particularly in diabetic wound healing.
To further validate the piezoelectric conversion capability of the prepared material, we tested its output voltage response under ultrasonic pressure. Figure 2f shows that both PLLA and PLLA@Ga exhibit distinct output voltage signals when subjected to dynamic US pressure, indicating that the material can effectively convert US energy into electrical energy, further confirming its excellent piezoelectric performance. In addition to the piezoelectric response tests, we also investigated the ability of PLLA@Ga to generate ROS under US stimulation. For this purpose, we employed electron spin resonance (ESR) spectroscopy to assess the generation of hydroxyl radicals (•OH) under US stimulation. As shown in Fig. 2g, a prominent •OH characteristic peak was observed in the ESR spectrum, indicating that the PLLA@Ga composite material can effectively produce hydroxyl radicals under US stimulation. This suggests that PLLA@Ga undergo specific reactions under US stimulation, converting hydrogen peroxide (H2O2) into highly reactive •OH radicals, thereby further enhancing the material’s antibacterial properties and promoting wound healing.
In summary, the experimental results indicate that the PLLA@Ga composite material not only possesses excellent piezoelectric performance but also enhances antibacterial effects through ROS generation under US stimulation. This broadens the application prospects of the material in diabetic wound healing, particularly in modulating immune responses, promoting wound repair, and preventing infections.
In this study, we immersed PLLA@Ga composites in PBS buffer solution (pH = 7.4) and recorded the concentration changes of Ga3+ in the solution at different time points, both in the presence and absence of US, as illustrated in Fig. 2h. Over the period from 6 h to 7 days, the concentration of gallium ions gradually increased, indicating that the PLLA@Ga material underwent an ion release process in the PBS solution. In comparative experiments, we observed that the Ga3+ release rate in the US (+) group was significantly higher than that in the US (-) group, with the total amount of Ga3+ at the end of the experiment also markedly greater in the US (+) group. This suggests that the application of ultrasound can effectively accelerate the release of Ga3+ from PLLA@Ga composites, likely due to mechanical effects induced by ultrasound that enhance the dissolution or diffusion rate of the ions. Furthermore, we investigated the impact of pH on Ga3+ release. Experimental results indicated that under lower pH conditions (pH = 5.5), the release rate of Ga3+ from PLLA@Ga was significantly accelerated. This phenomenon may be related to the increased degradation rate of the material in an acidic environment, facilitating the leaching of Ga3+ from the composite. Given that wound sites often exhibit localized acidic conditions, particularly during the inflammatory phase or in the presence of bacterial infection, lower pH values may promote the release of more Ga3+, thereby enhancing the antibacterial efficacy of the material.
Characterization of PLLA@Ga. (a) PFM image of PLLA@Ga material surface. (b) Piezoelectric amplitude of PLLA@Ga. (c) Phase angle of PLLA@Ga. (d) Butterfly cycle curve of PLLA@Ga. (e) Piezoelectric flip curve of PLLA@Ga. (f) The output voltage of PLLA@Ga under US stimulation. (g) ESR of PLLA@Ga with US or without US. (h) Ga3+ concentration was determined by ICP-OES after immersion of PLLA@Ga in PBS at different pH (pH 7.4 and pH 5.5) for different times with or without US
In vitro effects of PLLA@Ga + US
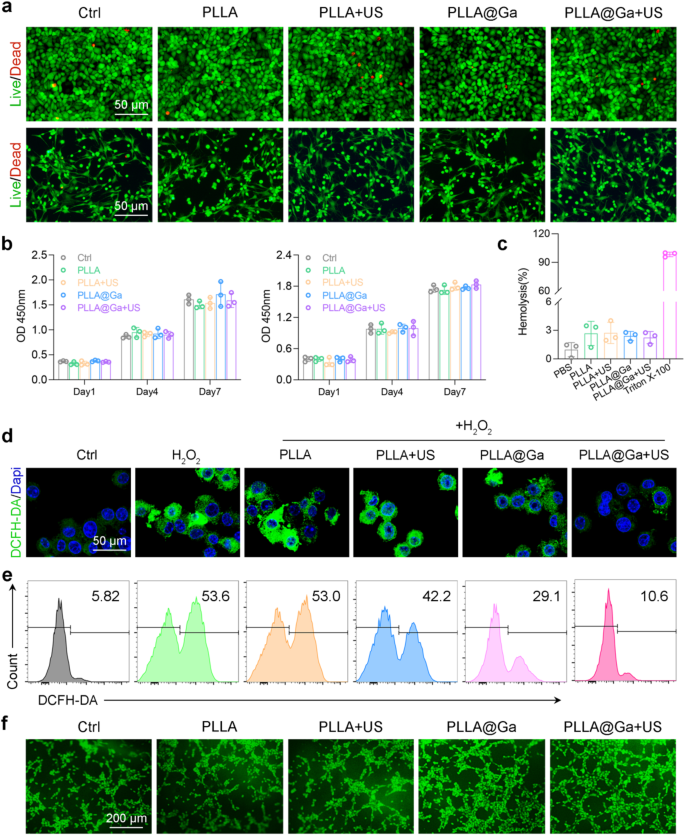
The importance of good biocompatibility cannot be overstated in the clinical application of biomaterials. To assess this, we conducted in vitro biocompatibility testing on various materials using live/dead staining, CCK8, and hemolysis assays. Initially, HUVEC and RS1 cells were cultured on different spinnerets with or without ultrasound stimulation. Following a 24-hour incubation period, live/dead staining was performed. The results depicted in Fig. 3a illustrate that after the treatments, the majority of HUVEC and RS1 cells exhibited high viability (green fluorescence) with minimal cell death (red fluorescence). These findings suggest that PLLA@Ga demonstrates biocompatibility and supports cell survival. The impact of PLLA@Ga on cell proliferation was further examined through CCK8 analysis. The CCK-8 assay results demonstrated that cell proliferation increased over the course of 1, 4, and 7 days of culture, indicating that PLLA@Ga supports cell growth without cytotoxicity (Fig. 3b). Unlike the 1-day culture period, cell absorbance values increased after 4 and 7 days of culture with all treatments, suggesting that PLLA@Ga effectively enhances cell proliferation. Hemolysis experiments also confirmed the blood compatibility of PLLA@Ga, as none of the biomaterial treatments resulted in significant hemolysis (Fig. 3c). These findings highlight the favorable biocompatibility, non-hemolytic nature, and support for cell adhesion, growth, and proliferation provided by PLLA@Ga composite electrospinning.
The typical characteristics of diabetic wounds include elevated levels of ROS, indicating that the wound is in a state of persistent oxidative stress. Immunofluorescence staining using DCFH-DA was employed to assess the ability of macrophages in the oxidative stress damage treatment group to clear intracellular. The control group exposed to H2O2 showed higher DCFH-DA staining, suggesting the induction of oxidative stress. Notably, after treatment with PLLA@Ga + US, the fluorescence signal decreased to levels similar to those of cells not exposed to H2O2 (Fig. 3d). The results are further supported by flow cytometry results (Fig. 3e). These findings suggest that PLLA@Ga + US can enhance the antioxidant capacity of macrophages by scavenging internal oxidants. Enhancing angiogenesis is crucial for tissue repair. Therefore, we employed the HUVEC tube formation assay to evaluate the impact of PLLA@Ga + US treatment on angiogenesis. The experimental results demonstrated a significant increase in the tubular network in the PLLA@Ga + US group compared to the control group (as shown in Fig. 3f), indicating that this treatment effectively promotes endothelial cell tube formation and the angiogenic process. The enhanced tube formation capability serves as a hallmark of endothelial cell migration, proliferation, and differentiation during angiogenesis, providing robust evidence for vascular reconstruction in the wound healing process. Furthermore, to validate the angiogenic potential of PLLA@Ga + US, we measured the secretion levels of vascular endothelial growth factor (VEGF) across different treatment groups using Elisa (Figure S2). VEGF is a crucial factor that promotes angiogenesis by stimulating endothelial cell proliferation and the formation of new blood vessels. The results revealed that the secretion levels of VEGF in the PLLA@Ga + US group were significantly higher than those in the control group, further substantiating the efficacy of this treatment in promoting angiogenesis.
Effects of PLLA@Ga + US in vitro. (a) Images of live/dead assays of HUVECs (upper panel) and RS-1 cells (lower panel). (b) CCK-8 assay of HUVECs (left panel) and RS-1 cells (right panel). (c) Hemolysis assay results of vatious materials exposure. (d) Fluorescence imaging was employed to assess the reduction of intracellular ROS in H2O2-stimulated Raw264.7 cells. (e) Analysis of intracellular ROS in H2O2-stimulated Raw264.7 cells after different treatments via flow cytometry. (f) Tube formation via coculturing HUVECs with various hydrogels
The observed increase in cell proliferation and high cell viability on PLLA@Ga membranes is consistent with previous studies demonstrating the biocompatibility of Ga-doped materials. For instance, Li et al. reported that Ga3+ enhance cell adhesion and proliferation by modulating the extracellular matrix environment [38]. Additionally, the improved hydrophilicity of PLLA@Ga, as evidenced by the reduced water contact angle, aligns with findings from Sun et al., who highlighted that hydrophilic surfaces promote cell attachment and growth [39]. The antioxidant capacity of PLLA@Ga, as demonstrated by the reduction in intracellular ROS levels, is also supported by studies showing that Ga3+ can scavenge ROS and mitigate oxidative stress [40]. These results collectively suggest that PLLA@Ga not only supports cell proliferation but also creates a favorable microenvironment for tissue regeneration.” The enhanced tube formation observed in the HUVEC assay indicates that PLLA@Ga promotes angiogenesis, a critical factor in diabetic wound healing. This finding is particularly significant given the impaired angiogenesis typically associated with diabetic wounds.
Antibacterial efficacy of PLLA@Ga + US
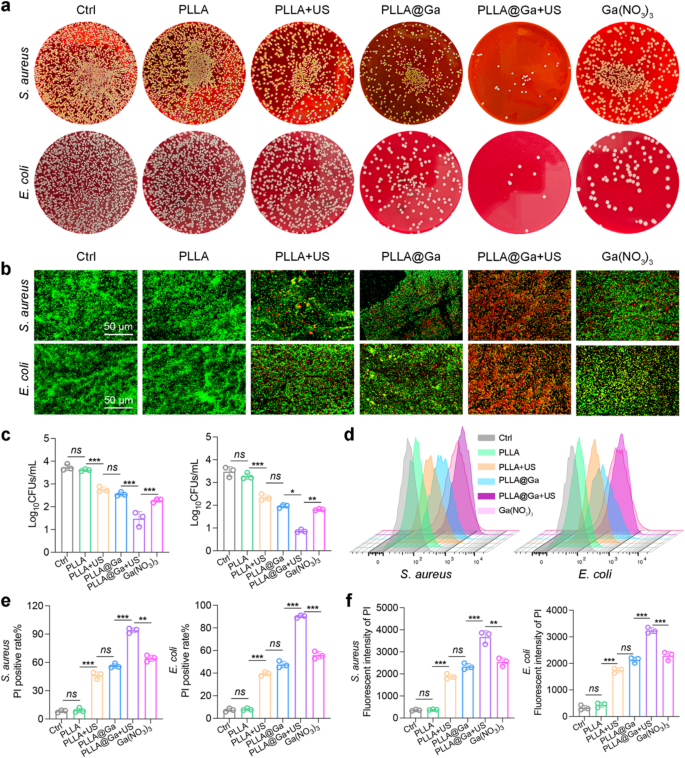
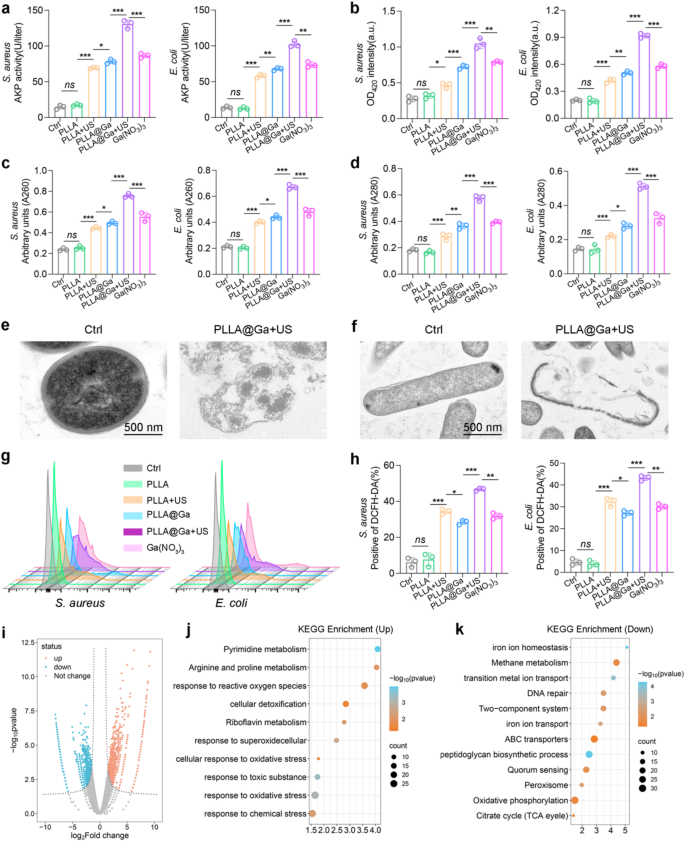
Dressings possessing potent antimicrobial properties are effective in safeguarding against pathogenic bacterial contamination, a critical factor influencing the progression of wound healing. The antibacterial effectiveness of PLLA@Ga + US electrospun fibers was assessed by testing them against various bacteria. The bacterial colonies were quantified post various treatments using SPM. As depicted in Fig. 4a and c, and S1, the bacterial colony count significantly decreased following the PLLA@Ga + US treatment. The bacteria subjected to various treatments were stained using live/dead staining and examined under a fluorescence microscope. The findings illustrated in Fig. 4b reveal a substantial increase in bacterial mortality (red fluorescence) within the PLLA@Ga + US group in comparison to other treatments, accompanied by a notable decrease in the number of surviving bacteria (green fluorescence). These outcomes underscore the potent antibacterial effectiveness of PLLA@Ga + US.
Subsequently, PI staining was employed to assess the bactericidal capability of PLLA@Ga + US (Fig. 4d). For S. aureus, the PI fluorescence intensity of the PLLA@Ga + US group increased by 9.45-fold, 9.13-fold, 1.26-fold, 0.76-fold, and 0.64-fold compared to the control group, PLLA group, PLLA + US group, PLLA@Ga group, and Ga(NO3)3 group (Fig. 4e), respectively. Similarly, for E. coli, the PI fluorescence intensity of the PLLA@Ga + US group increased by 9.89-fold, 9.13-fold, 1.41-fold, 0.87-fold, and 0.72-fold relative to the control group, PLLA group, PLLA + US group, PLLA@Ga group, and Ga(NO3)3 group (Fig. 4e), respectively. This indicates that the PLLA@Ga + US treatment can significantly disrupt bacterial cell membranes, enhancing their permeability. Furthermore, quantitative analysis of PI fluorescence intensity using a microplate reader corroborated the results obtained from the aforementioned flow cytometry (Fig. 4f). The results showed a significant correlation between PLLA@Ga + US treatment and increased PI fluorescence intensity, highlighting its potential as an effective antibacterial strategy.
In vitro antibacterial activity of PLLA@Ga + US. (a) Representative plates of bacteria colonies after treatment with different samples. (b) The fluorescence images of live (green) and dead bacterial (red). (c) Results of bacteria treated with various materials that formed colonies after 24 h. (d-e) Representative flow cytometry plots and quantitative analysis of PI. (f) Detection of membrane damage in bacteria through monitoring PI influx. Data are presented as mean ± SD (n = 3). “ns” represent “no significant”; *P < 0.05; **P < 0.01; ***P < 0.001
Antibacterial efficacy of PLLA@Ga + US
Furthermore, the antibacterial effect of PLLA@Ga + US was further investigated by assessing the extracellular AKP content and ONPG hydrolysis assay. Following treatment with PLLA@Ga + US, there was a significant increase in extracellular AKP concentration (Fig. 5a), indicating damage to the bacterial cell wall. This discovery suggests that this therapy is capable of effectively disrupting the bacterial cell wall. ONPG enters the cytoplasm and is subsequently hydrolyzed by cytoplasmic β-galactosidase, indicating an increase in bacterial cell membrane permeability. Notably, the absorbance at 420 nm significantly increased following PLLA@Ga + US treatment (Fig. 5b), suggesting enhanced bacterial cell membrane permeability. Compared to other groups, bacteria treated with PLLA@Ga + US exhibited a significant increase in nucleic acid and protein absorbance at 260 nm and 280 nm (Fig. 5c-d). These results demonstrate that PLLA@Ga + US treatment can significantly alter bacterial membrane permeability, leading to cytoplasmic leakage. Furthermore, TEM results revealed that bacteria treated with PLLA@Ga + US showed contracted, blurred, and corrugated cell membranes, whereas the control group displayed smooth, intact, and well-defined cell membranes (Fig. 5e).
Subsequently, the potential mechanism of PLLA@Ga + US sterilization was investigated. According to previous studies, piezoelectric materials can produce a large amount of ROS under ultrasonic stimulation. Therefore, we speculate that this treatment may exhibit antibacterial properties through ROS activity. As shown in Figs. 5g–h, bacteria treated with PLLA@Ga + US exhibited a significant increase in ROS production. Therefore, ROS plays an integral role in the mechanism of PLLA@Ga + US sterilization. Furthermore, as per previous reports, Ga3+ possesses antibacterial activity primarily because bacteria absorb Ga and bind it to iron-dependent enzymes [41, 42]. However, Ga3+ is unable to be reduced under physiological conditions, rendering Ga-substituted enzymes unable to carry out the tasks of their iron-dependent counterparts, which are essential for bacterial growth. Since bacteria cannot differentiate between the two, they are unable to reduce their intake of Ga3+, as doing so would also decrease their intake of iron. Therefore, the potent bactericidal effect of PLLA@Ga + US can be attributed to the significant amount of Ga3+ disrupting bacterial iron metabolism and ultimately leading to bacterial death.
Next, RNA sequencing analysis was conducted on surviving S. aureus to identify gene expression differences between the control group and the PLLA@Ga-treated group under US treatment. Volcano plots were produced to visually represent the differentially expressed genes observed between the Ctrl group and the PLLA@Ga + US group (Fig. 5i). Utilize the KEGG enrichment method to analyze differentially expressed genes. In the PLLA@Ga + US group, genes associated with ROS, superoxide, and oxidative stress were significantly upregulated (Fig. 5j), indicating that PLLA@Ga + US treatment can enhance oxidative stress in S. aureus. Previous studies have shown that Ga3+ can bind to bacterial cytochromes, inhibiting the electron transfer of cytochrome-bound oxygen, leading to ROS production and inducing bacterial death [43]. Therefore, PLLA@Ga + US may inhibit bacterial growth by enhancing bacterial oxidative stress. In addition, pathways related to DNA repair are significantly downregulated in the PLLA@Ga + US group (Fig. 5k). This suggests that DNA repair is effectively inhibited, catalytic reactions within the bacteria are disrupted, and bacterial morphological regulation is suppressed. Genes related to iron ion transport were significantly downregulated in the PLLA@Ga + US group (Fig. 5k), which can be explained by the bacteria’s inability to distinguish between Ga3+ and Fe3+, resulting in the uptake of a large amount of Ga3+ [44]. Iron is one of the most crucial nutrients for bacteria, involved in essential intracellular redox and electron transfer processes. Consequently, PLLA@Ga + US can inhibit bacterial growth by interfering with S. aureus’s iron metabolism.
These results indicate that PLLA@Ga + US can inhibit the iron ion transport of S. aureus, reduce the activity of bacterial antioxidant enzymes, and amplify the oxidative stress of bacteria, thereby enhancing the antibacterial activity of PLLA@Ga during the SDT process.
In vitro antibacterial activity of PLLA@Ga + US. (a) The sensitivity to alkaline phosphatase activity. (b) The ONPG hydrolysis activity. (c-d) Leakage of cell contents after exposure to various treatments was investigated by monitoring the absorbance of extracellular material at 260 nm (A260) and 280 nm (A280) corresponding to nucleic acids and proteins, respectively. (e-f) Morphology of bacteria from different treatment groups as observed by TEM. (g) Assessment of ROS generation was conducted using DCFH-DA fluorescent probes by FCM. (h) Assessment of ROS generation was conducted using DCFH-DA fluorescent probes by microplate reader. (i) Volcano map of differentially expressed genes (DEGs). (j-k) KEGG pathway enrichment analysis. Data are presented as mean ± SD (n = 3). “ns” represent “no significant”; *P < 0.05; **P < 0.01; ***P < 0.001
Macrophage behavior
Numerous research findings indicate that the efficiency of wound healing is closely tied to the local inflammatory reaction within the tissue [45,46,47]. Undoubtedly, the immune system significantly influences this intricate process. Overactive or dysregulated immune responses can worsen tissue damage and impede the healing process. Hence, optimal wound healing and restoration transpire within a harmonious and well-regulated immune environment. Macrophages are recognized for their crucial involvement in the immune system and have the ability to transform into two distinct phenotypes, M1 (pro-inflammatory subtype) and M2 (pro-repair subtype), depending on the stimuli they encounter. M1 macrophages encourage the development of an inflammatory microenvironment through the secretion of various pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), IL-6, and IL-1β. Conversely, M2 macrophages facilitate tissue repair by releasing cytokines like IL-4, CD206, and IL-10 to establish an anti-inflammatory setting [48, 49]. The significance of this polarization is paramount, as the appropriate polarization of M1 and M2 macrophages greatly improves the tissue repair process. Conversely, excessive activation and polarization can worsen inflammation and hinder efficient wound healing [50]. Undoubtedly, the host immune response is a crucial factor in the wound healing process facilitated by biomaterials. Given the critical nature of these immune responses, our focus now shifts to investigating the immunomodulatory impacts of PLLA@Ga + US and its potential to enhance wound healing through modulation of macrophage activities.
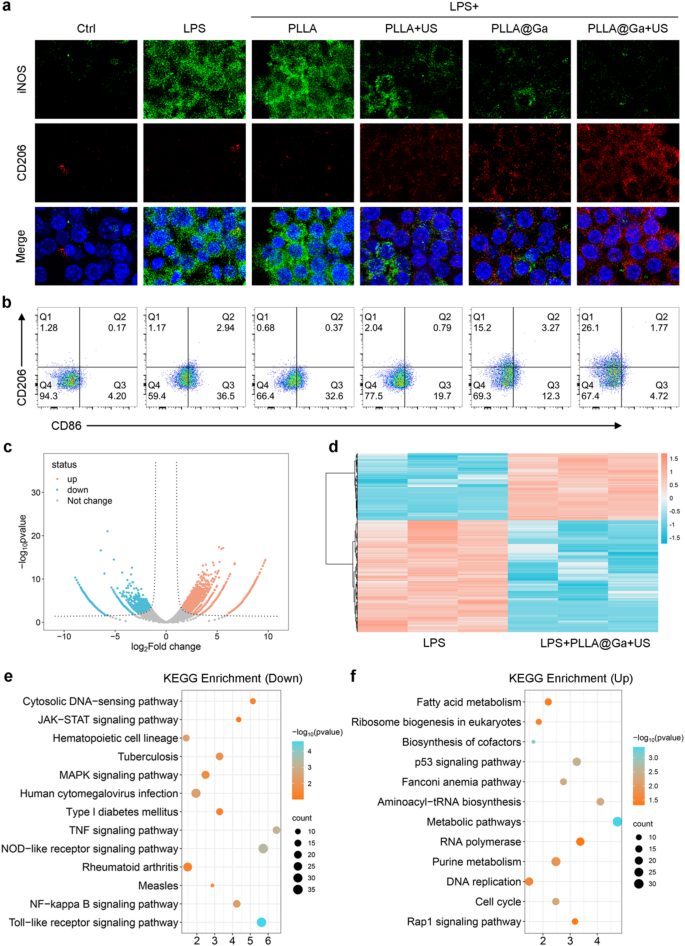
Macrophages were seeded on different fibrous membranes for 24 h under LPS-induced inflammation. The immunomodulatory effect of PLLA@Ga + US was evaluated by analyzing the expression of macrophage M1 and M2 subtype markers through immunofluorescence staining. Results depicted in Fig. 6a revealed that after exposure to LPS, macrophages exhibited elevated levels of iNOS (green fluorescence). Conversely, treatment with PLLA + US, PLLA@Ga, and PLLA@Ga + US, particularly the latter, significantly decreased iNOS expression, suggesting an inhibitory effect on its expression (Figure S3a). Furthermore, the fluorescence intensity of CD206 notably increased following treatments with PLLA + US, PLLA@Ga, and PLLA@Ga + US compared to LPS treatment. Notably, PLLA@Ga + US exhibited the most pronounced effect on CD206 fluorescence intensity (Figure S3b). These findings indicate that PLLA@Ga + US has the potential to counteract the LPS-induced inflammatory microenvironment, suppressing macrophage M1 polarization while promoting M2 polarization.
The impact of different treatments on macrophage polarization was subsequently evaluated through flow cytometry analysis. The results revealed a significant decrease in the percentage of CD86-positive cells (M1 marker) and a notable increase in CD206-positive cells (M2 marker) following treatment with PLLA + US, PLLA@Ga, and PLLA@Ga + US, particularly PLLA@Ga + US. Specifically, the distribution of CD86-positive cells in the control, LPS, LPS + PLLA, LPS + PLLA + US, LPS + PLLA@Ga, and LPS + PLLA@Ga + US groups were 4.20%, 36.5%, 32.6%, 19.7%, and 12.3%, and 4.72%, respectively, while the proportions of CD206-positive cells were 1.28%, 1.17%, 0.68%, 2.04%, 15.2%, and 26.1% (Fig. 6b). These outcomes suggest a close relationship between PLLA@Ga + US treatment and the shift of macrophages towards the anti-inflammatory M2 phenotype. The downregulation of CD86 and upregulation of CD206 highlight the ability of PLLA@Ga + US to direct macrophages towards the reparative M2 subtype, which is vital for effective wound healing in cases of infection.
Furthermore, an in-depth investigation into the effects of PLLA@Ga + US on macrophages was conducted using RNA-Seq. Differentially expressed genes (DEGs) were identified with a p-value of less than 0.05 and a fold change (FC) greater than 2. The volcano plot in Fig. 6c vividly illustrates the substantial number of DEGs between the PLLA@Ga + US treatment group and the LPS group. The heatmap constructed based on these differential genes highlights the changes in gene expression between the LPS group and the LPS + PLLA@Ga + US group (Fig. 6d). KEGG pathway enrichment analysis further revealed specific signaling pathways associated with macrophage phenotype transformation (Fig. 6e-f). The analysis indicated that after PLLA@Ga + US treatment, inflammatory pathways related to M1 macrophage polarization, including MAPK signaling pathway, TOR-like receptor signaling pathway, NF-kappa B signaling pathway, and Nod-like signaling pathway, were significantly inhibited. In addition, upregulation of anti-inflammatory pathways was observed, particularly those associated with M2 macrophage polarization, such as the TGF-β signaling pathways. This change in gene expression suggests that macrophages may be reprogrammed towards a more reparative phenotype, thereby promoting tissue regeneration.
To validate the RNA-Seq findings, the impact of PLLA@Ga + US treatment on the expression of inflammation-related genes in macrophages was examined using qPCR. After 24 h of culture, the expression levels of typical M1 macrophage markers (including IL-1β, TNF-α, and iNOS) in the PLLA@Ga + US treatment group were found to be significantly lower compared to those in the LPS treatment group alone (Figure S1c). Conversely, the expression level of the M2 macrophage marker IL-10 showed a significant increase, which aligns with the RNA-Seq results. Elisa was employed to measure the secretion of inflammatory factors. The PLLA@Ga + US treatment demonstrated a significant decrease in the secretion levels of pro-inflammatory cytokines like IL-1β and TNF-α (Figure S1d). These findings provide additional evidence supporting the ability of PLLA@Ga + US treatment to modulate macrophage transformation towards the anti-inflammatory subtype. These findings highlight the therapeutic potential of PLLA@Ga + US in modulating macrophage behaviour, thereby influencing the overall inflammatory response and promoting a healing-favourable microenvironment.
The characteristics of macrophages are influenced by various factors such as their inducers, properties, and surface markers. This research involved the creation of electrospun PLLA@Ga with piezoelectric capabilities to investigate its impact on macrophage phenotype. The findings indicated that applying PLLA@Ga with ultrasonic stimulation resulted in a reduction of M1-type macrophages induced by LPS, while promoting an increase in M2-type macrophages. By comparing with ICP results, we found that the concentration of Ga ions in PLLA@Ga is 11.74 mg/L. Previous studies have demonstrated that free Ga ions can inhibit the expression of inflammatory genes [51], indicating that Ga ions play an indispensable role in the anti-inflammatory effects of PLLA@Ga. Additionally, several research has shown that the piezoelectric effect induced by gentle US also possesses certain anti-inflammatory properties [30], which has been further confirmed in this study.
Studies on the in vitro immunomodulatory of PLLA@Ga + US. (a) Expression of iNOS and CD206 following different hydrogel treatments. (b) Analysis of CD86 and CD206 in macrophages following various treatments via flow cytometry. (c) Volcano map of differentially expressed genes (DEGs). (d) Heat map of differentially expressed genes (DEGs). (e) KEGG enrichment for the down-regulated pathways. (f) KEGG enrichment for the up-regulated pathways
In vivo wound healing of PLLA@Ga + US
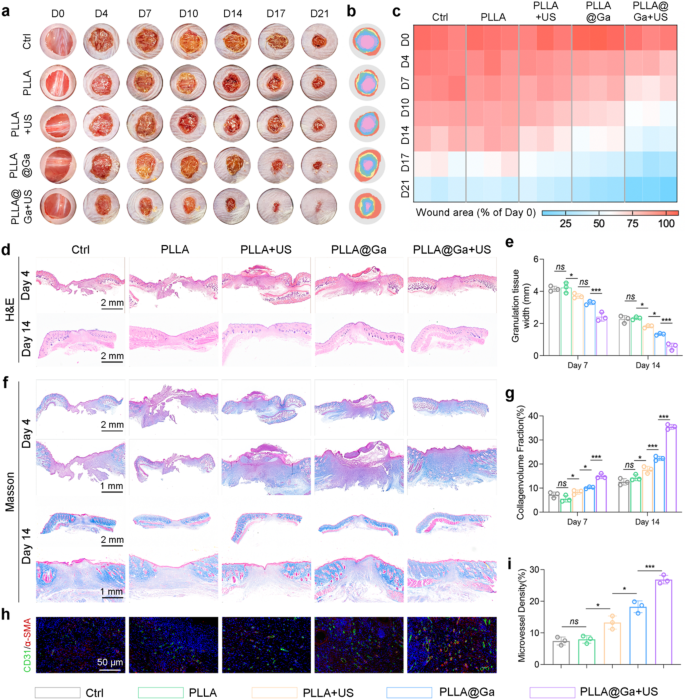
In the context of diabetes-related wounds, prolonged delays in healing pose a heightened risk of bacterial infection, thereby impairing the body’s immune response and impeding the wound recovery process. This scenario can potentially escalate to severe infections and critical health complications [52, 53]. Hence, expediting the wound healing process is crucial for both preventing and managing infections. This research delves into exploring the impact of PLLA@Ga on wound healing within an infected diabetic mouse model. Mice were induced with diabetes by injecting STZ and creating a 10 mm full-thickness wound on mice with blood glucose levels exceeding 16.65 mmol/L, followed by contamination with bacteria. Wounds treated with saline (control group), PLLA, PLLA + US, PLLA@Ga, and PLLA@Ga + US were photographed and evaluated on days 0, 4, 7, 10, 14, 17, and 21. As shown in Fig. 7a-b, wounds treated with PLLA@Ga + US healed faster compared to other treatment groups. The healing rates of wounds treated with PLLA@Ga + US on days 4, 7, 10, 14, 17, and 21 were 21.5%, 36.9%, 48.2%, 57.3.73%, and 93.7% respectively, while the control group only achieved a healing rate of 65.2% on day 21 (Fig. 7c).
On the 7th and 21st day post-operation, histological examination of the infected wound skin was conducted using H&E and Masson’s trichrome staining for tissue analysis. Quantitative assessment of granulation tissue length, as shown in Fig. 8d-e, indicated a significantly accelerated wound contraction rate in the PLLA@Ga + US treatment group compared to the others. Adequate collagen deposition is crucial for tissue strength and healing during the remodeling process. We employed Masson’s trichrome staining to evaluate new collagen. By day 21, the control group still had residual scabs, indicating delayed healing (Fig. 8f). In contrast, wounds treated with PLLA@Ga + US exhibited a clear red epidermal layer and more collagen. Furthermore, PLLA@Ga + US treatment also enhanced chronic wound healing, promoting effective regeneration of dermal appendages (Fig. 8g). In conclusion, the study findings suggest that PLLA@Ga + US treatment enhances the healing rate of infection-related diabetic wounds compared to other groups. Research findings indicate that successful wound healing relies on angiogenesis to provide crucial nutrients and oxygen. Thus, sufficient angiogenesis plays a vital role in the wound healing process [54]. The study assessed the presence of neoangiogenic markers, specifically cell adhesion molecule 1 (CD31) and α-smooth muscle actin (α-SMA), in sections of mouse skin wounds (Fig. 8h-i). The results revealed notably elevated levels of CD31 and α-SMA in wounds treated with PLLA@Ga + US, underscoring their noteworthy potential for promoting wound healing.
PLLA@Ga + US promotes diabetic wound healing. (a-b) Photographs of wounds at predetermined time points and superimposed images of wounds after various treatments. (c) Areas of unhealed wounds in each group at different time intervals. (d) H&E staining. (e) Corresponding quantitative analysis of the granulation tissue width. (f) Masson’s trichrome staining. (g) Statistical analysis of collagen deposition during the remolding phase. (h) Representative immunofluorescence staining of microvessel of wound. (i) Statistical analysis of microvessel density. Data are presented as mean ± SD (n = 3). “ns” represent “no significant”; *P < 0.05; ***P < 0.001
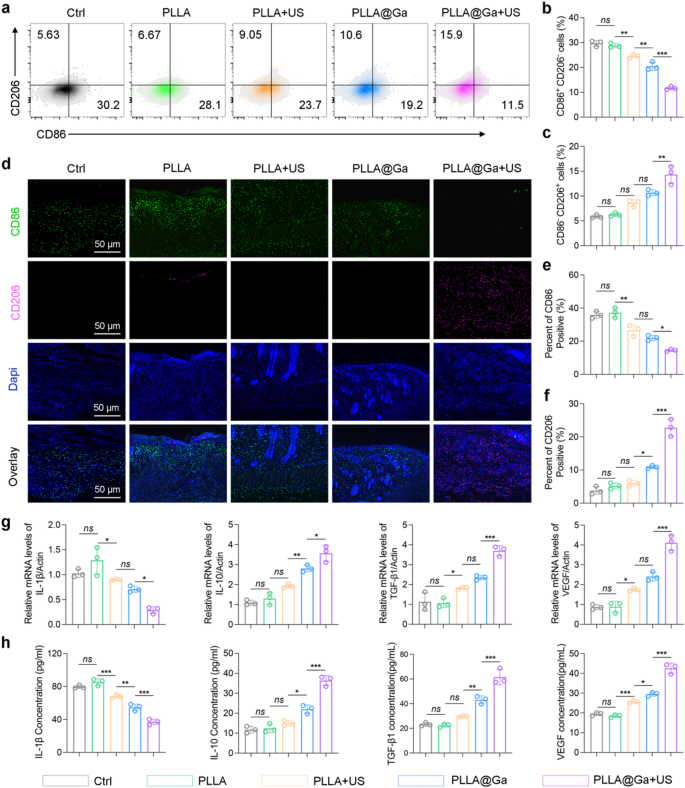
The effective treatment for diabetic infected wounds involves not only rapidly eradicating bacteria but also reversing the inflammatory response in the diabetic microenvironment. Histological analysis indicates that PLLA@Ga + US treatment can achieve rapid healing of diabetic infected wounds. In vitro experiments demonstrate that PLLA@Ga + US treatment can inhibit macrophage M1 polarization and promote M2 polarization. Therefore, it is reasonable to speculate that PLLA@Ga + US treatment can reverse the inflammatory microenvironment of diabetic wounds in vivo, thereby promoting wound healing. Collect wounds subjected to different treatments and analyze the single-cell suspensions using flow cytometry to quantify macrophage populations. Figure 8a-c demonstrate that, compared to other treatment groups, the PLLA@Ga + US treatment group exhibited fewer M1-like macrophages (CD86+CD206−) and a significant increase in M2-like macrophages (CD86−CD206+). Immunofluorescence staining of tissue-associated M1 (CD86) and M2 (CD206) macrophages revealed that PLLA@Ga + US treatment significantly enhanced M2 macrophage markers while reducing the expression of M1 macrophage markers (Fig. 8d-f). These findings confirm that PLLA@Ga + US treatment improves the local immune microenvironment of diabetic infected wounds by decreasing CD86+ M1 macrophages and increasing CD206+ M2 macrophages near the wound, which is more conducive to wound healing. Moreover, PCR and Elisa were employed to quantify the gene expression and protein secretion levels of M1 cell factors (related to the pro-inflammatory marker IL1β) and M2 cell factors (anti-inflammatory and angiogenesis markers IL10, TGFβ1, and VEGF) (Fig. 8g-h). The analysis results revealed a significant decrease in M1 macrophages factor expression levels and an increase in M2 macrophages factor protein levels in the PLLA@Ga + US treatment group, surpassing other treatment groups. These findings indicate that PLLA@Ga + US can effectively promote the healing of diabetic infected wounds.
Macrophages play a crucial role in the immune response to tissue damage. In diabetic wound infections, the transition of macrophages from an inflammatory (M1) to an anti-inflammatory (M2) state is disrupted, impeding wound healing [55, 56]. Our in vitro and in vivo studies demonstrate that PLLA@Ga + US effectively reduces the number of M1 macrophages while increasing the number of M2 macrophages, thereby enhancing the expression of anti-inflammatory and angiogenic cytokines, including IL-10, VEGF, and TGF-β1. Specifically, IL-10 is a cytokine that alleviates inflammation and inhibits oxidative processes. Vascular endothelial growth factor plays a critical role in recruiting endothelial cells, thereby initiating angiogenesis. Additionally, TGF-β1 recruits endothelial cells and fibroblasts to the wound site, promoting the synthesis of ECM [57]. In conclusion, PLLA@Ga + US shows significant potential in accelerating the healing of diabetic-infected wounds. By effectively modulating macrophage phenotypes, PLLA@Ga + US aids in suppressing inflammation response, promoting angiogenesis, and tissue remodeling.
Impact of the PLLA@Ga + US on macrophage polarization in vivo. (a-c) Flow cytometry of M1and M2 macrophages retrieved from the wound tissue. (d) Immunofluorescence staining of tissue sections at the wound site for CD86 and CD206. (e-f) Statistical analysis concerning the proportion of CD86 (f) and CD206 (g) positive macrophages. (g) Gene expression levels of IL-1β, IL-10, VEGF, and TGF-β1 in wound tissues. (h) Secretion of IL-1β, IL-10, VEGF, and TGF-β1 in wound tissues assessed by Elisa. Data are presented as mean ± SD (n = 3). “ns” represent “no significant”; *P < 0.05; **P < 0.01; ***P < 0.001
In this study, we evaluated the effects of PLLA + US treatment on major organs, including the heart, liver, spleen, lungs, and kidneys, through H&E staining. The results indicated that none of these organs exhibited significant toxic responses following PLLA@Ga + US treatment (Figure S4). Specifically, the staining images did not reveal any tissue damage, inflammation, or other toxic characteristics. The tissue architecture of the heart, liver, spleen, lungs, and kidneys remained intact, with normal cellular morphology and no evident cellular infiltration or tissue destruction. Furthermore, the blood biochemical parameters of mice, such as total protein (TP), albumin (ALB), globulin (GLO), total bilirubin (TBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, and glucose (GLU), showed no significant changes after different treatments (Figure S5), indicating that PLLA@Ga + US possesses excellent biocompatibility and biosafety. These findings suggest that PLLA@Ga + US treatment demonstrates good biocompatibility with these vital organs and does not elicit toxic responses, thereby providing a safety assurance for its clinical application. Furthermore, the ultrasound treatment did not cause any damage to the organs, further validating that the combined action of PLLA composites and ultrasound can promote wound healing while maintaining systemic safety.
Despite the promising in vitro and in vivo results, several challenges remain for the clinical translation of PLLA@Ga. These include the scalability of the electrospinning process, the long-term stability of the piezoelectric properties, and the potential need for repeated ultrasound treatments in clinical settings. Additionally, the safety and efficacy of PLLA@Ga in human patients need to be rigorously evaluated through clinical trials. It is important to note that while PLLA@Ga shows promising results, its performance should be contextualized within the broader field of advanced wound dressings. For instance, silver-based dressings and hydrogels have been widely used for their antibacterial properties, while growth factor-loaded scaffolds have shown efficacy in promoting angiogenesis [58,59,60]. Compared to these materials, PLLA@Ga offers a unique combination of piezoelectricity and Ga ion release, which may provide synergistic benefits in modulating the immune microenvironment and promoting tissue repair. However, further studies are needed to directly compare the efficacy of PLLA@Ga with these existing materials.