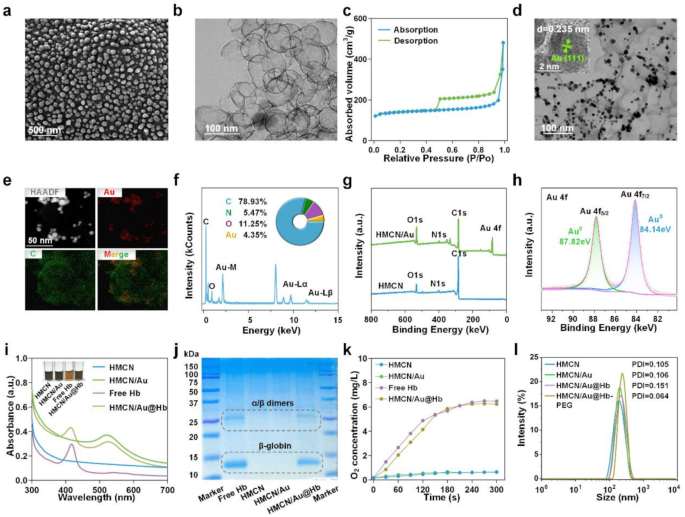
Preparation and characterization of HMCN/Au@Hb
Initially, HMCN was fabricated as a core–shell structure using a modified hard-templating method. Scanning electron microscopy (SEM) images revealed that HMCN were spherical, uniformly distributed, and had an average diameter of 57.06 ± 10.2 nm (Fig. 1a). Transmission electron microscopy (TEM) further demonstrated that the HMCN possessed an inner hollow structure alongside with an average ultrathin shell thickness of 2.35 nm (Fig. 1b). Brunauer Emmet Teller (BET) N2 absorption-desorption analysis confirmed the presence of abundant voids with pore size of 1.76 nm and a high specific surface area up to 532.7475 m2/g, contributing to the enhanced loading capacity of HMCN (Fig. 1c and Figure S1). After physical absorption of Au onto the surface of HMCN, Au presented as protrusions in TEM images, like chocolate beans on cookies. Au and HMCN were thus tightly integrated and HMCN/Au was successfully fabricated (Fig. 1d). Furthermore, the lattice plane spacing of 0.235 nm measured through the high-resolution TEM (HRTEM) image corresponds to the (111) diffraction plane of Au (Fig. 1d insert). Simultaneously, the successful decoration of Au was proved by mapping element profiles (Fig. 1e), and the coexistence of Au and C elements in the HMCN/Au were further validated by energy-dispersive X-ray spectrometer (EDS) (Fig. 1f). Afterwards, X-ray photoelectron spectroscopy (XPS) was performed to measure the element composition and valence states of HMCN/Au (Fig. 1g). The high-resolution Au 4f spectrum can be deconvoluted into two doublets centered at 87.82 and 84.14 eV, labeled as Au4f5/2 and Au4f7/2, respectively, which indicated that Au precursor was partially reduced to metallic Au (Fig. 1h) [36]. In addition, the selected area electron diffraction (SAED) pattern with the (111), (200), (220) and (311) planes of cubic HMCN/Au echo the results from HRTEM (Figure S2).
Preparation and characterization of HMCN/Au@Hb. (a) SEM and (b) TEM images of HMCN. (c) N2 absorption/desorption isotherms of HMCN. (d) TEM images of HMCN/Au (Insert was HRTEM). (e) HAADF-STEM image and elemental mapping for HMCN/Au. (f) Quantitative elemental analysis of HMCN/Au conducted using EDS. The inserts were the comprised element and corresponding atomic frequencies. (g) XPS analysis of the HMCN and HMCN/Au. (h) The high-resolution XPS spectra of Au 4f for HMCN/Au. (i) UV-VIS spectra of free Hb, HMCN, HMCN/Au and HMCN/Au@Hb, respectively (Insert was the color change before and after loading). (j) Coomassie blue staining performed on electrophoresed acrylamide loaded with Hb. (k) Oxygen releasing properties measured by RDPP probe. (l) Size distribution of HMCN, HMCN/Au, HMCN/Au@Hb and PEGylated HMCN/Au@Hb determined by DLS.
Given to the hollow and mesopores of HMCN/Au, Hb was efficiently loaded and HMCN/Au@Hb was eventually formed. The UV-VIS spectra of HMCN/Au@Hb exhibited characteristic absorption peaks at 410 and 525 nm corresponding to Hb and Au (Fig. 1i). Moreover, the insert image illustrates the color changes during the synthesis of HMCN/Au@Hb, the dark gray HMCNs turned into a slightly brownish dark grey color after loaded with Hb (Fig. 1i insert). Coomassie blue staining revealed protein bands at 14 and 25 kDa, in correlation with the β-globin and α/β dimers of Hb (Fig. 1j) [37]. Based on the standard concentration curve of Hb (Figure S3a, b), the optimal mass ratio was determined to be 1:5 (Hb: HMCN) (Table S1). The oxygen-carrying capacity of different nanomaterials was then assessed using a dissolved-oxygen meter. In particular, a significant rise in O2 levels was detected in free Hb and HMCN/Au@Hb, which confirmed that Hb retained its oxygen-carrying activity during the synthesis process (Fig. 1k). Moreover, the surface of HMCN/Au@Hb were modified with DSPE-PEG2000-NH2 to enhance the biocompatibility and stability in physiological condition. Dynamic light scattering (DLS) analysis showed a slightly increased particle size following Au decoration, Hb loading and PEGylation. The hydrodynamic sizes of HMCN, HMCN/Au, HMCN/Au@Hb and PEGylated HMCN/Au@Hb dispersed in deionized water were measured as 177.57 ± 5.10 nm (PDI = 0.105), 205.50 ± 2.50 nm (PDI = 0.106), 228.67 ± 3.05 nm (PDI = 0.151) and 259.77 ± 4.95 nm (PDI = 0.064), respectively (Fig. 1l), with a uniform size distribution. In summary, the successful preparation of HMCN/Au@Hb was confirmed through element composition determination, morphological observation and spectral characterization.
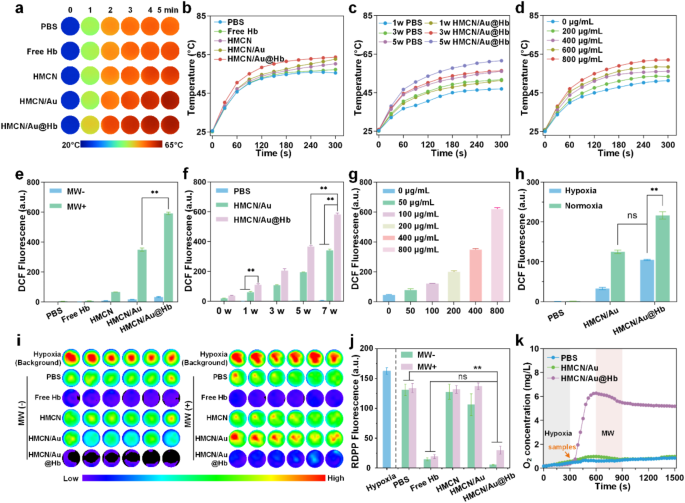
Microwave responsive and oxygen carrying capacities of HMCN/Au@Hb
Considering HMCN exhibits high dielectric constants due to its unique porous structure and conductive carbon framework, the microwave-thermal performance of HMCN/Au@Hb was monitored under controlled microwave parameters, including power density and nanocomposites concentration [38]. Following a 5-min microwave irradiation (3.0 W), the control and free Hb group only increased to 55.5 and 57.2℃, respectively. While under the same irradiation condition, HMCN, HMCN/Au and HMCN/Au@Hb (400 µg/mL) were heated up to 60.2, 62.6 and 63.7℃, respectively, the final temperature of HMCN/Au@Hb was about 8.2℃ higher than the temperature of PBS. These results demonstrate that HMCN/Au@Hb contributed to enhancing microwave-to-heat conversion efficiency to a certain extent (Fig. 2a) (corresponding infrared thermal image was presented in Fig. 2b). Additionally, HMCN/Au@Hb was irradiated with microwave under different power densities, the temperature and the microwave power density were positively correlated (Fig. 2c) (corresponding infrared thermal image was presented in Figure S4a). Moreover, upon exposure to microwave (3.0 W), the temperature increased from 51.3 to 53.4, 56.1, 58.4 and 62.0℃ with increasing concentration of HMCN/Au@Hb (0, 200, 400, 600 and 800 µg/mL) (Fig. 2d), indicating that the microwave-thermal effect of HMCN/Au@Hb was proportional to the sample concentration (corresponding infrared thermal image was presented in Figure S4b). These findings highlight the potential of HMCN/Au@Hb as an excellent microwave absorption agent to improve the microwave-thermal conversion efficiency for tumors.
Microwave responsive and oxygen carrying capacities of HMCN/Au@Hb. (a) Real-time infrared thermal images and (b) corresponding heating profiles of different samples (400 µg/mL) under microwave irradiation (3 W, 5 min). (c) Microwave heating profiles of PBS and HMCN/Au@Hb (400 µg/mL) under microwave irradiation at different power densities for 5 min. (d) Microwave heating profiles of HMCN/Au@Hb at different concentrations under microwave irradiation (3 W, 5 min). (e) ROS detection of different samples (200 µg/mL) irrespective of microwave irradiation (7 W, 5 min). (f) ROS detection of different samples (200 µg/mL) under microwave irradiation (5 min) at different power levels. (g) ROS detection of HMCN/Au@Hb suspensions at different concentrations under microwave irradiation (3 W, 5 min). (h) ROS detection of different samples (PBS, HMCN/Au and HMCN/Au@Hb) at the concentration of 200 µg/mL under hypoxic or normoxic conditions under microwave irradiation (3 W, 5 min). (i) Fluorescence images and (j) quantitative analyses of different samples showing oxygen generation under different conditions. (k) Dynamic oxygen generation of different samples under microwave irradiation (3 W, 5 min). Data were presented as mean values ± SD (n = 3). (* represents p<0.05, ** represents p<0.01)
Inspired by the strong SPR characteristic of Au and the sufficient oxygen released by Hb, we inferred that Au could generate active or “hot” electrons so as to induce electron’s transition upon microwave irradiation and further absorb oxygen molecules as sustainable fuel for ROS generation [39]. To investigate whether the HMCN/Au@Hb possessed the ability of microwave-dynamic performance, the production and species determination of ROS under microwave irradiation were performed through electron spin resonance (ESR). Notably, the HMCN/Au@Hb + MW group exhibited a characteristic sextet signal corresponding to superoxide anions (•O2−), which was remarkably higher than the HMCN/Au + MW group. The result indicated that Hb might play a vital role in oxygen-amplified microwave-dynamic performance (Figure S5). We then used the DCFH-DA probe to detect total ROS generation of HMCN/Au@Hb. Upon microwave irradiation (7.0 W), the fluorescence intensity of HMCN/Au@Hb was almost 1.7 times higher than that of HMCN/Au, indicating the positive effect of oxygen in amplifying ROS generation (Fig. 2e). Subsequently, the fluorescence intensity of PBS, HMCN/Au and HMCN/Au@Hb showed a similarly power-dependent increase. Under the same power density, the HMCN/Au@Hb group exhibited greater fluorescence intensity compared to that of the HMCN/Au group (Fig. 2f). Moreover, the ROS generation of HMCN/Au@Hb showed a concentration-dependent trend (Fig. 2g). Therefore, we believed that the oxygen carried by HMCN/Au@Hb facilitated the amplification of ROS generation. After irradiated with 3.0 W microwave, the ROS signals of HMCN/Au in normoxic environment was 1.7 times higher than that in hypoxic environment, but comparable to HMCN/Au@Hb in the same hypoxic environment (Fig. 2h). The result demonstrated that Hb might supplement the O2 content in hypoxic environment, which would further facilitate the oxidation process.
To further explore the inner oxygen-carrying property of HMCN/Au@Hb, we employed an oxygen indicator, RDPP probe, which can be quenched by molecular oxygen, to visually investigate the oxygen production [40]. Notably, the fluorescence intensity in the PBS and HMCN groups displayed a negligible change. However, the fluorescence intensity in both the free Hb and HMCN/Au@Hb group weakened dramatically, suggesting the similarly superior O2 generation activities of both the free Hb and HMCN/Au@Hb (Fig. 2i), which was consistent with the corresponding quantitative data presented in Fig. 2j. Besides, both PBS and HMCN/Au contained very little oxygen and remained unchanged after irradiating with microwave, whereas HMCN/Au@Hb (3 mg/mL) showed immediate increase in the O2 concentration at a hypoxic solution and maintained the extremely high level of O2 during the process of microwave irradiation (Fig. 2k). The above results confirmed the oxygen-carrying activity of HMCN/Au@Hb, as well as a small amount of oxygen consumption to generate ROS under the exposure of microwave.
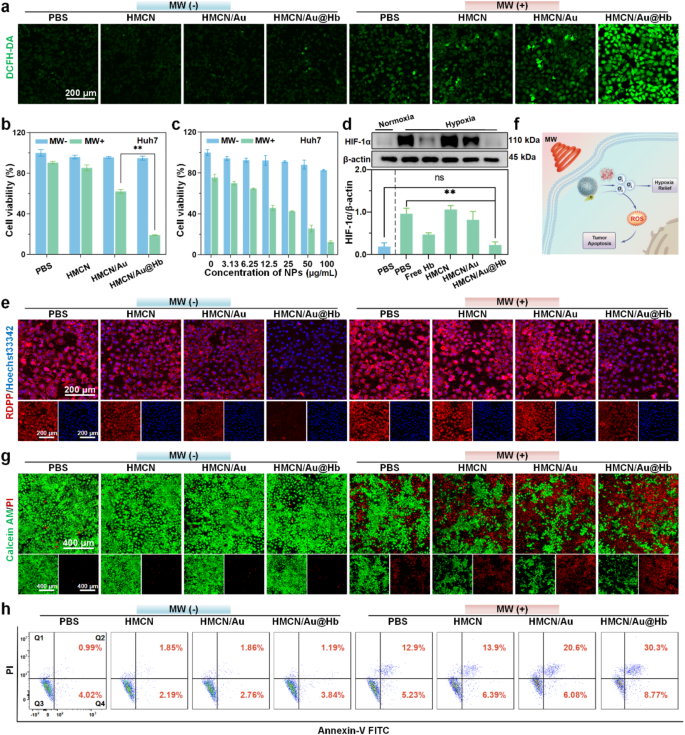
Cytotoxicity of HMCN/Au@Hb under microwave irradiation
Inspired by the ROS generation property at the solution level, we further evaluated HMCN/Au@Hb induced ROS outbreak at the cellular level. DCFH-DA probe was utilized to visualize and characterize the ROS generation via confocal laser scanning microscope (CLSM) [41]. Herein, invisible green fluorescence was detected in cells treated with PBS or NPs alone, while more intense green fluorescence was shown upon microwave irradiation. Moreover, the brightest green fluorescence was observed in the HMCN/Au@Hb + MW group (Fig. 3a). Furthermore, the quantitative data across different groups found that the ROS levels in the HMCN/Au@Hb + MW group were approximately 1.8-fold higher than that of the HMCN/Au + MW group, indicating that intrinsic oxygen-carrying capacity of Hb might induce a greatly enhanced microwave-dynamic effect (Figure S6a). Subsequently, the aforementioned cells were harvested for flow cytometry analysis, and showed a consistent trend compared to those observed in DCFH-DA assay (Figure S6b). These findings directly highlighted the effectiveness of HMCN/Au@Hb in generating ROS triggered by microwave irradiation. We further utilized upper transwell chamber to mimic “transition zone” to investigate whether tumor cells in the marginal “transition zone” could still cause ROS burst under ultra-low microwave irradiation, Although the temperature in the upper chamber of the microwave irradiation group was significantly lower than that in the bottom chamber and direct heating group, the ROS levels were comparable to those in the direct heating group. It further demonstrated that even under low-power microwave irradiation in the “transition zone”, nanoparticles could still be effectively excited to produce ROS (Figure S7).
In vitro microwave-induced cytotoxic effect. (a) CLSM images of intracellular ROS level with different treatments (green, fluorescence of DCFH-DA). (b) Cytotoxicity of Huh-7 cells treated with different samples and (c) treated with HMCN/Au@Hb at different concentrations. (d) Western blot demonstrating the HIF-1α expression levels with different treatments. (e) CLSM images of intracellular O2 level with different treatments (blue, fluorescence of Hoechst 33342; red, fluorescence of RDPP; overlay images). (f) Schematic illustration of HMCN/Au@Hb mediated microwave thermal-dynamic effect. (g) Live/Dead co-staining and (h) Annexin V/PI staining of Huh-7 cells after different treatments. Data were presented as mean values ± SD (n = 3). (* represents p<0.05, ** represents p<0.01)
Given that excessive oxidative stress detrimentally impacts the cellular killing effect, the antitumor effect of HMCN/Au@Hb in Huh-7 cells was initially characterized by MTT assay [42]. After being irradiated with microwave, the cell viability rate reveals an approximately 80% reduction in MW + HMCN/Au@Hb (50 µg/mL) group, which was higher than that in MW + HMCN/Au (50 µg/mL) group (Fig. 3b). Especially, cell viability was gradually reduced in the HMCN/Au@Hb + MW group with increasing concentrations of HMCN/Au@Hb, supporting its effectively antitumor effect in a concentration-dependent manner. By contrast, insignificant cytotoxic effect was observed in non-irradiated conditions, suggesting the noncytotoxic nature of HMCN/Au@Hb (Fig. 3c). To further investigate the cytotoxicity of HMCN/Au@Hb in normal cell lines, human normal hepatic cell (LO2 and MIHA) and renal cell (293T) were used in the following experiments. Even at an extremely high concentration of 400 µg/mL, 3 types of cell line remained viability of 76.4%, 85.6% and 80.7%, respectively, indicating the commendable biosafety of HMCN/Au@Hb (Figure S8).
Due to the sufficient O2 released by HMCN/Au@Hb, we speculated that the inherent oxygen levels would substantially elevate and mitigate the constraints imposed by the hypoxic microenvironment, thereby facilitating ROS production and amplifying the therapeutic effect [43]. Preliminary investigation of the hypoxia-relieving effect in vitro, western blot was conducted to detect the expression level of HIF-1α [44]. HIF-1α expression level was significantly down-regulated in the HMCN/Au@Hb group but comparable to that of the negative control (PBS under normoxic conditions), solidly verifying that both Hb and HMCN/Au@Hb possessed equal hypoxia-allaying effects (Fig. 3d). Subsequently, to investigate the dynamic process of oxygen variation, cellular O2 content was analyzed by CLSM observation using the RDPP indicator. After incubated with HMCN/Au@Hb, the red fluorescence intensity was weakened acutely compared to that of the PBS group, suggesting a substantial increase in cellular oxygen levels. Nevertheless, the red fluorescence in the HMCN/Au@Hb + MW group was slightly stronger than that in the HMCN/Au@Hb group (Fig. 3e). Fluorescence semi-quantitative (Figure S9a) and flow cytometry measurements (Figure S9b) further confirmed that oxygen consumption may occur through generating ROS upon microwave stimulus. Altogether, owing to the specific hypoxic nature of tumor tissue, we speculated that the sufficient oxygen released by Hb-loaded HMCN/Au might facilitate ROS generation in vitro, thereby ameliorating the hypoxic microenvironment and augmenting the therapeutic efficacy (Fig. 3f) [45].
In addition, the antitumor effect induced by excessive ROS and microwave hyperthermia was assessed using Calcein-AM/PI co-staining. Ultralow cell death rates were observed in non-irradiated groups. After irradiation, cell death rates increased dramatically, and cells incubated with HMCN/Au@Hb exhibited the highest death rate, which was conformed to the findings of the MTT assay (Fig. 3g). The fluorescence quantitative results also remarkably emphasized the significant outcomes of the MW coupling system (Figure. S10). Consequently, cell apoptosis assay was performed on Huh-7 cells to identify the type of apoptosis triggered by ROS and hyperthermia. Cells treated with HMCN/Au + MW and HMCN/Au@Hb + MW displayed a substantial increase in the proportion of apoptotic (Annexin V-positive) and necroptotic cells (PI-positive) (Fig. 3h). Especially, obvious apoptosis signals (Q2 + Q4 quadrant: 39.1%) were observed in the HMCN/Au@Hb + MW group, which was superior to that of the HMCN/Au + MW group (Q2 + Q4 quadrant: 26.7%) (Figure. S11).
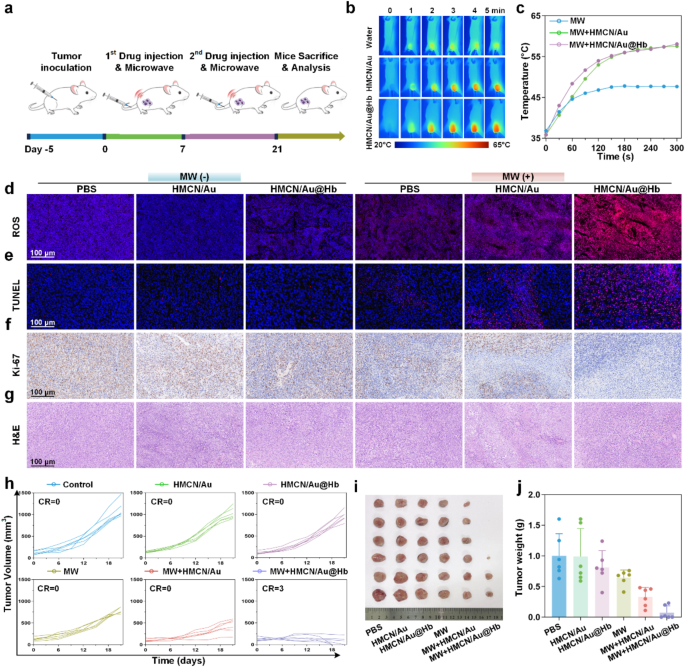
In vivo antitumor effect
Due to the remarkable in vitro cell death effect, the in vivo antitumor effects were evaluated on tumor-bearing mice. The HCC models were randomly divided into six groups (n = 6): PBS, HMCN/Au, HMCN/Au@Hb and their respective MW groups. Figure 4a showed the entire in vivo experimental process. Throughout the treatment, in vivo infrared thermal images (Fig. 4b) and corresponding temperature curves (Fig. 4c) revealed that tumor temperature in the MW + HMCN/Au and MW + HMCN/Au@Hb groups increased rapidly within 5 min, even reached 58.1 °C and 57.6 °C under an ultralow microwave power density (3.0 W), respectively. However, the temperature of tumors without NPs administration only increased to 47.7 °C, indicating that HMCN/Au and HMCN/Au@Hb exhibited an excellent microwave-thermal effect in vivo due to their similar localized heat accumulation ability. To further evaluate the biodistribution of HMCN/Au@Hb, ICG labeled fluorescence imaging was performed on Hepa1-6 tumor xenograft mice. Following intravenous injection of free ICG or HMCN/Au@Hb@ICG, fluorescence images were acquired at different timepoints (0, 1, 2, 4, 6, 8, 12 and 24 h). Both groups exhibited strong fluorescence signal at 1 h (Figure S12a). Notably, HMCN/Au@Hb demonstrated significantly higher fluorescence retention at the tumor site compared to free ICG, with stable tumor fluorescence persisting beyond 24 h, whereas the ICG group showed minimal signal beyond 8 h. This prolonged tumor accumulation was likely attributed to the optimal nanoparticle size, facilitating the enhanced permeability and retention (EPR) effect (Figure S12b). Ex vivo imaging further confirmed sustained fluorescence in tumors, supporting the prolonged retention of HMCN/Au@Hb in tumor tissue (Figure S12c). In addition, we supplemented the Au content in tumor and other organs with Inductively Coupled Plasma Mass Spectrometry (ICP-MS), it revealed that obvious Au enrichment in the tumor of HMCN/Au@Hb group at the time of sacrifice (1-hour post-injection), demonstrating the excellent tumor accumulation potential. Besides, it also demonstrated significant hepatic and renal uptake, presumably due to the primary involvement of the liver and kidneys in the metabolic clearance of the nanomaterials (Figure S13). However, ex vivo distribution of both free ICG and ICG-labeled HMCN/Au@Hb decreased rapidly and was nearly completely cleared after 24 h administration, as previously shown in Figure S12c.
In vivo antitumor effect. (a) Schematic illustration of the experimental schedule for the subcutaneous tumor model treatments. (b) Infrared thermal images and (c) the corresponding heating profiles of tumor-bearing mice during microwave irradiation. Histological analysis of sacrificed tumor tissues after a 21-day treatment (d) ROS, (e) TUNEL, (f) Ki-67 and (g) H&E staining. (h) Individual of tumor growth curves in different groups. (i) Photographs (j) and the corresponding weight of tumor tissues at 21st day after treatments. Data were presented as mean values ± SD (n = 6). (* represents p<0.05, ** represents p<0.01)
The potent antitumor capability of HMCN/Au@Hb was further investigated through ROS, TUNEL, Ki-67 and H&E staining of tumor tissues dissociated on day 21. Significant variations in the morphology of tumors were observed across different treatment groups. Remarkably, HMCN/Au@Hb + MW treated tumors exhibited higher levels of ROS generation than the other groups, indicating that ROS outbreak may play a pivotal role in tumor suppression (Fig. 4d, Figure S14a). In addition, TUNEL staining showed that HMCN/Au@Hb + MW group exhibited the most intense red fluorescence, indicative of apoptotic tumor cell, highlighting the superior microwave-dynamic property to induce cancer cell apoptosis (Fig. 4e, Figure S14b). Similarly, tumors stained with Ki-67 (Fig. 4f, Figure S14c) and H&E (Fig. 4g) revealed that HMCN/Au@Hb + MW displayed the lowest tumor cell proliferation and the most extensive karyorrhectic debris, in sharp contrast to the abundant proliferation and limited necrosis observed in other groups. These findings demonstrated that HMCN/Au@Hb achieved pronounced antitumor effect by inducing ROS outbreak upon microwave irradiation.
Encouraged by the great performance of HMCN/Au@Hb in thermal imaging and tumor cell damage in vivo, changes in tumor volume were monitored throughout 21-day treatment (Figure S15). Compared to the rapidly tumor growth in the control, HMCN/Au, HMCN/Au@Hb and MW-only groups, only moderate tumor inhibition observed in the HMCN/Au + MW group, while the tumor growth curves began to increase starting on the 15th day, since the insufficient oxygen may produce inadequate microwave-dynamic effect and ultimately lead to incomplete tumor necrosis and recurrence. Meanwhile, the HMCN/Au@Hb + MW group presented with substantial tumor suppression, as well as a significant and definitive curative effect (Fig. 4h and Figure S16). Tumor photographs (Fig. 4i) and tumor weights (Fig. 4j) explained the similar result in a more intuitive and clear way. Furthermore, the potential side effects were evaluated during the 21-day observation period. Notably, no significant fluctuations were observed in body weight across all diverse groups, which preliminarily confirmed the excellent biosafety of all treatments in mice (Figure S17). To further investigate potential thermal damage to normal tissues adjacent to tumor lesions post-ablation, we conducted pathological examinations. Histological analyses revealed a well-demarcated ablation zone, and there were no obvious heating damage effects in surrounding muscle tissues upon microwave irradiation (Figure S18). Moreover, H&E staining was performed on major organs across different treated mice, and no observable tissue necrosis was found. It suggested that both nanomaterials administration and microwave irradiation had no visible pathological abnormalities or inflammatory lesions in vivo (Figure S19). Collectively, HMCN/Au@Hb exhibited superior tumoricidal effect and favorable biosafety, which was expected to be a biocompatible nanomaterial for microwave ablative therapy in the future.
To investigate the tumor recurrence, we further conducted a various of experiment groups to continuously monitor the tumor growth for 33 days (Note: tumor volume ≥ 1500 mm3 defined as dead). Comparative analysis at day 7 post-ablation revealed that the MW + HMCN/Au@Hb group (V/V0 = 0.998) showed better tumor inhibition effect than the MW-alone group (V/V0 = 1.555). Notably, some selected tumor-bearing mice even achieved complete tumor ablation in MW + HMCN/Au@Hb group. In contrast, the tumor volume in the non-ablation group surpassed the ethical endpoint threshold (≥ 1500 mm3), necessitating termination of observation due to rapid progression (Figure S20a). More encouragingly, there was no significant local recurrence in the MW + HMCN/Au@Hb group even at day 33 post-ablation (V/V0 = 0.414), in sharp contrast to the MW group (V/V0 = 7.481) (Figure S20b). In addition, the survival rate of mice treated with HMCN/Au@Hb plus MW was significantly prolonged (Figure S20c). Therefore, compared to microwave ablation alone, HMCN/Au@Hb significantly enhanced the efficacy of microwave ablation and effectively inhibited tumor recurrence at post-ablation.
Antitumor immune response
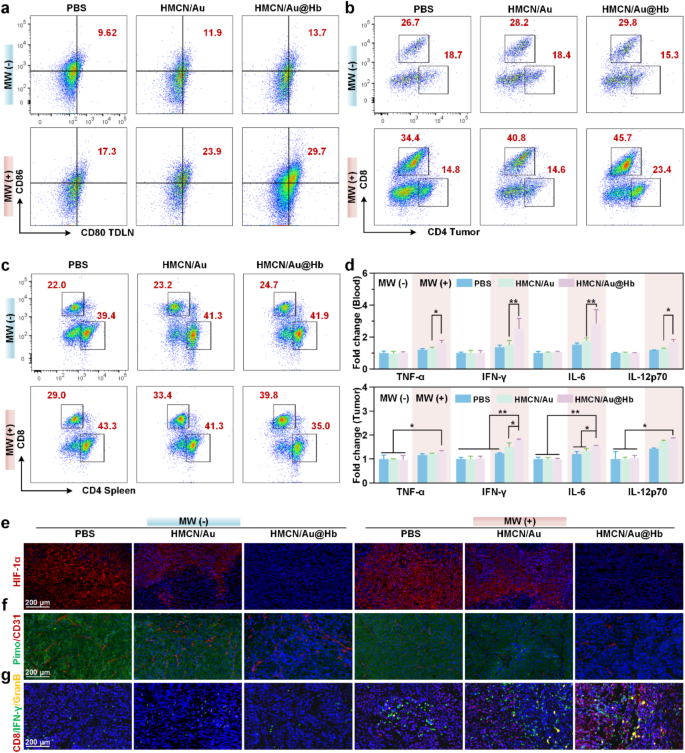
To further investigate the underlying mechanism of the antitumor effect of HMCN/Au@Hb, the activation of systemic immunity was explored [46]. Previous studies have shown that DCs, mostly contained in tumor draining lymph nodes (TDLNs), acted as potent antigen-presenting cells (APCs) for capturing tumor associated antigens (TAAs) [47]. Herein, we found that the HMCN/Au@Hb + MW group showed a greater matured DCs (CD80+CD86+) infiltration, which far exceeded that observed in the HMCN/Au + MW group (Fig. 5a). Quantitative data further confirmed these findings (Figure S21a). The results implied that Hb-loaded HMCN/Au mediated microwave-dynamic effect appeared to facilitate antigen presentation to DCs and trigger downstream immune response. Given to its important role in promoting DC maturation, the differentiation of T lymphocytes within the primary tumor were examined [48]. Notably, the greater CD8+ T cells infiltration were evoked by microwave stimulus and HMCN/Au@Hb administration (Fig. 5b), and the higher intratumoral CD8+/CD3+ T cell ratios were observed in irradiated groups compared with non-irradiated groups, particularly in combination with HMCN/Au@Hb (Figure S21b). Next, we performed flow cytometry analysis in splenic homogenates of mice to investigate the activation of T cells in immune organs. The percentage of CD8+ T cells were significantly elevated in the HMCN/Au@Hb + MW group compared to the other groups (Fig. 5c). Likewise, higher CD8+/CD3+ T cell ratio were observed in the HMCN/Au@Hb + MW group than that of the HMCN/Au + MW group (Figure S21c). These findings suggested that the oxygen-amplified microwave-dynamic effect enabled infiltration of immune cells to a larger extent.
Antitumor immune response. (a) Flow cytometry of DC maturation in TDLNs with indicated treatments. (b) Flow cytometry of T-cell activation in tumors and (c) spleens with indicated treatments. (d) ELISA analysis of the TNF-α, IFN-γ, IL-6, and IL-12p70 levels in serum and tumors. Immunofluorescence images of (e) HIF-1α, (f) Pimonidazole and CD31+, (g) CD8, IFN-γ and Granzyme B exposure in tumors. Data were all presented as mean values ± SD (n = 3). (* represents p<0.05, ** represents p<0.01)
Moreover, the role of various cell-secreted pro-inflammatory factors, such as TNF-, IFN-, IL-6, and IL-12p70, all of which had a vital function in modulating immune responses, were further explored via ELISA assay [49]. ELISA assay portrayed an elevated serum secretion levels of 1.28-, 1.68-, 1.61- and 1.33-fold for TNF-, IFN-, IL-6 and IL-12p70 in HMCN/Au@Hb + MW group than that in the HMCN/Au + MW group, respectively. As for intratumoral secretion levels, HMCN/Au@Hb upon microwave irradiation yielded the greatest TNF-, IFN-, IL-6 and IL-12p70 secretions, whereas their respective secretions in other groups were relatively lower, confirming that HMCN/Au@Hb plus MW could exert a robust activated-inflammatory effect (Fig. 5d).
Aiming to deepen our comprehension of the interaction between hypoxic relief and intrinsic antitumor immunity of HMCN/Au@Hb in vivo, immunofluorescence experiments were then performed on mouse tumor tissues. The specific hypoxic status of tumors was evaluated by HIF-1α expression levels [50]. Apparently, a significant down-regulation was observed in the HMCN/Au@Hb + MW group compared to the HMCN/Au + MW group (Fig. 5e, Figure S22). Meanwhile, accumulating evidence has shown that insufficient microwave treatment could aggravate hypoxia and induce angiogenesis in tumor sites, pimodazole and CD31+ staining were adopted for detecting hypoxic regions and microvessels [51]. Compared with the MW group, a dramatically reduction of green fluorescence (pimodazole staining) and red fluorescence (CD31+ staining) were observed in the HMCN/Au@Hb + MW group (Fig. 5f, Figure S22). It suggested the improved oxygenation and less angiogenesis at the tumor site, which would potentially contribute to reversing the immune-suppressive TME. Immunofluorescence analysis for CD8+, IFN-γ and Granzyme B further verified the increased infiltration of effector cytotoxic CD8+ T lymphocytes in tumor sites [47]. CD8+, IFN-γ and Granzyme B infiltration in combination therapy groups were greater than that of the monotherapy groups, and the HMCN/Au@Hb + MW group showed the strongest fluorescence intense (Fig. 5g, Figure S22). These findings confirmed the efficiency of HMCN/Au@Hb in converting naïve T cells into effector T cells (CD8+, IFN-γ and Granzyme B). Overall, the above findings demonstrated that intrinsic oxygen-carrying capacity of Hb induced a greatly enhanced microwave-dynamic effect. Altogether, HMCN/Au@Hb had the unique ability of in situ oxygen generation and maintain in vivo hypoxia-relieving effect, which benefits for reversing the immune-suppressive microenvironment. This study could pave the way towards expediting tumoricidal immunity.